P7E.6 Before attempting these calculations, see Problem P7E.5. The following data give the wavenumbers (wavenumbers in cm") of the v = 0 → 1 transition of a number of diatomic molecules. Calculate the force constants of the bonds and arrange them in order of increasing stiffness. Use integer relative atomic masses. 'H*CI 'H'Br 'H"I 2990 2650 2310 2170 1904
P7E.6 Before attempting these calculations, see Problem P7E.5. The following data give the wavenumbers (wavenumbers in cm") of the v = 0 → 1 transition of a number of diatomic molecules. Calculate the force constants of the bonds and arrange them in order of increasing stiffness. Use integer relative atomic masses. 'H*CI 'H'Br 'H"I 2990 2650 2310 2170 1904
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
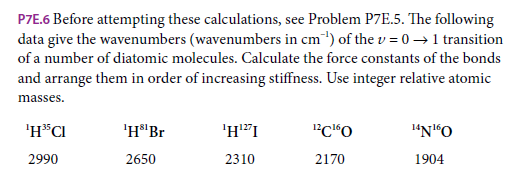
Transcribed Image Text:P7E.6 Before attempting these calculations, see Problem P7E.5. The following
data give the wavenumbers (wavenumbers in cm") of the v = 0 → 1 transition
of a number of diatomic molecules. Calculate the force constants of the bonds
and arrange them in order of increasing stiffness. Use integer relative atomic
masses.
'H*CI
'H'Br
'H"I
2990
2650
2310
2170
1904
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,