Part 2. For each salt solution, give the estimated pH and the indicators you used to determine the pH Salt Indicator I Indicator 2 Indicator 3 Estimated pH Indicator 4 Alizarin Yellaw methyl Orange Brimthml Cesl ed NH.CI Blue PH410.1 3.1 PHS4.4PH6.5 PH>72 Alizana Brumgtl Blue methy Cneal Red NasPO. Yellaw oranye H스10.1 PH>8,% 3.1 PH544 4 n27.4 Mathy! Orang Bronghh gl Bue Cresal ked yellow
Part 2. For each salt solution, give the estimated pH and the indicators you used to determine the pH Salt Indicator I Indicator 2 Indicator 3 Estimated pH Indicator 4 Alizarin Yellaw methyl Orange Brimthml Cesl ed NH.CI Blue PH410.1 3.1 PHS4.4PH6.5 PH>72 Alizana Brumgtl Blue methy Cneal Red NasPO. Yellaw oranye H스10.1 PH>8,% 3.1 PH544 4 n27.4 Mathy! Orang Bronghh gl Bue Cresal ked yellow
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter15: Acid-base Equilibria
Section: Chapter Questions
Problem 86E: A solution has a pH of 4.5. What would be the color of the solution if each of the following...
Related questions
Question
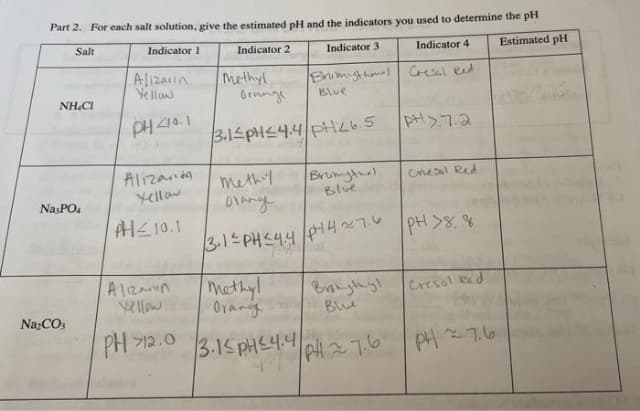
Transcribed Image Text:Part 2. For each salt solution, give the estimated pH and the indicators you used to determine the pH
Salt
Indicator 1
Indicator 2
Indicator 3
Estimated pH
Indicator 4
Alizarin
Ye llaw
methyl
Orange
Brumythml Cresal eed
NH.CI
Blue
PH410.1
pH>7.2
3.1 PHS4.4PH26.5
Alizanda
Yellow
methy
oranye
Brumgtul
Blue
Cnesl Red
NasPO
H스10.1
3.1 PHC44
47 PH >8. %
A1izarın
yellow
Mmethyl
Orang
Brongth yl
Bue
Cresol Bed
Na:CO:
PH 기2.0
3.15 PHS4.4
PH27.6 PH 76
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning