Part A If the second reaction is carried out with an 85.0 % yield, what mass of titanium can be obtained from 1.00 kg of the ilmenite-sand mixture? Express your answer with the appropriate units. HA ? Value Units Submit Request Answer Provide Feedback Next
Part A If the second reaction is carried out with an 85.0 % yield, what mass of titanium can be obtained from 1.00 kg of the ilmenite-sand mixture? Express your answer with the appropriate units. HA ? Value Units Submit Request Answer Provide Feedback Next
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter13: Electrochemistry
Section: Chapter Questions
Problem 13.103PAE
Related questions
Question
Transcribed Image Text:M Review | Constants | Periodic Table
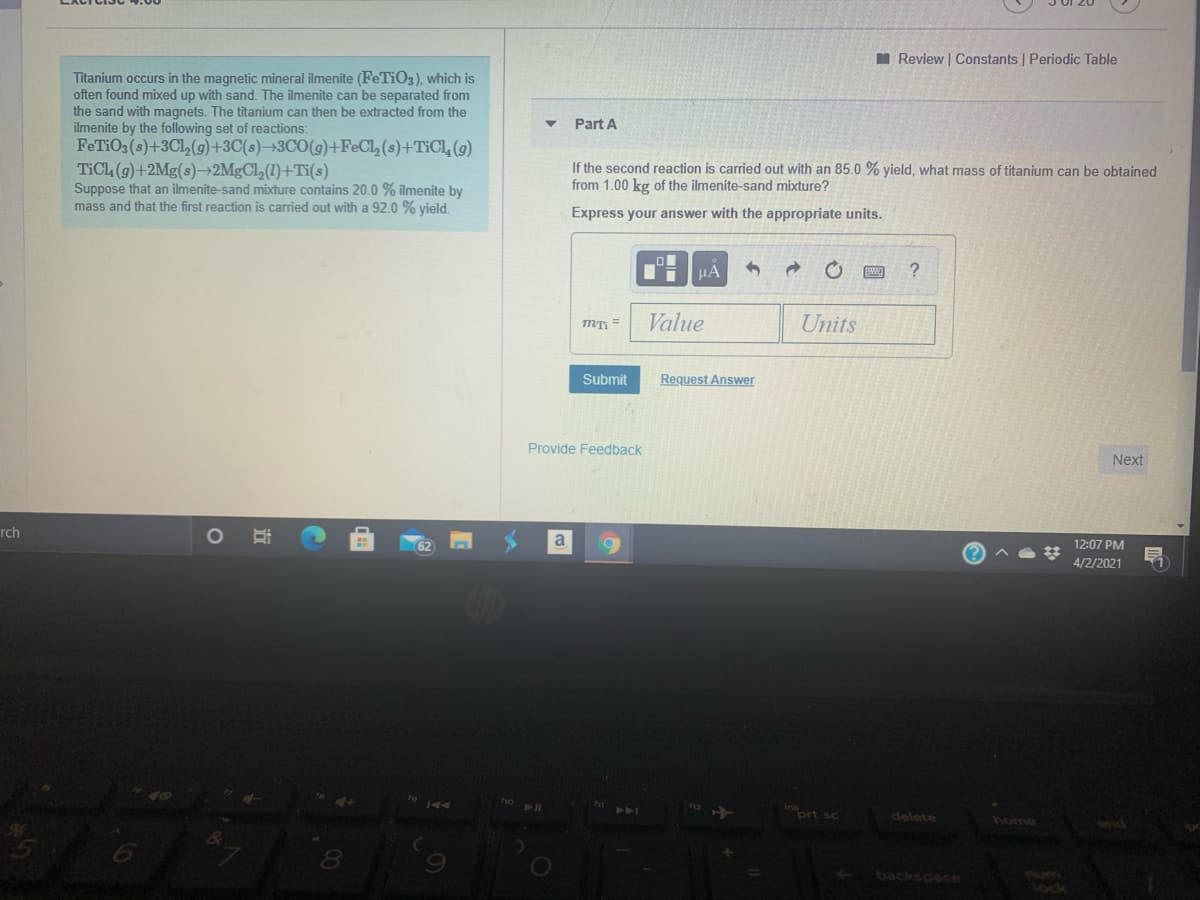
Titanium occurs in the magnetic mineral ilmenite (FeTiO3), which is
often found mixed up with sand. The ilmenite can be separated from
the sand with magnets. The titanium can then be extracted from the
ilmenite by the following set of reactions:
FeTiO3 (s)+3Cl,(g)+3C(s)→3CO(g)+FeCl, (s)+TIC1, (9)
TICL (9)+2Mg(s)–2MgCl,(1)+Ti(s)
Suppose that an ilmenite-sand mixture contains 20.0 % ilmenite by
mass and that the first reaction is carried out with a 92.0 % vield.
Part A
If the second reaction is carried out with an 85.0 % yield, what mass of titanium can be obtained
from 1.00 kg of the ilmenite-sand mixture?
Express your answer with the appropriate units.
HA
Value
Units
MTI =
Submit
Request Answer
Provide Feedback
Next
rch
62
12:07 PM
4/2/2021
K4
411
ingrt sc
AA
delete
home
8
backspace
oum
Sock
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning