VISUALIZATION Determining an Empirical Formula Carbon-Hydrogen Analysis Unit Furnace HOabsorber Co, absorber 0.400 g vitamin C 00:25 00:27 During the analysis, 0.00905 mol H,O is formed. Calculate the amount (mol) H in 0.00905 mol H2O. 0.00905 mol H,0 formed x mol Recheck Next (2 of 5) 8th attempt Incorrect
VISUALIZATION Determining an Empirical Formula Carbon-Hydrogen Analysis Unit Furnace HOabsorber Co, absorber 0.400 g vitamin C 00:25 00:27 During the analysis, 0.00905 mol H,O is formed. Calculate the amount (mol) H in 0.00905 mol H2O. 0.00905 mol H,0 formed x mol Recheck Next (2 of 5) 8th attempt Incorrect
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 4E
Related questions
Question
H2O absorber: 0.163g H2O
CO2 absorber: 0.600g CO2
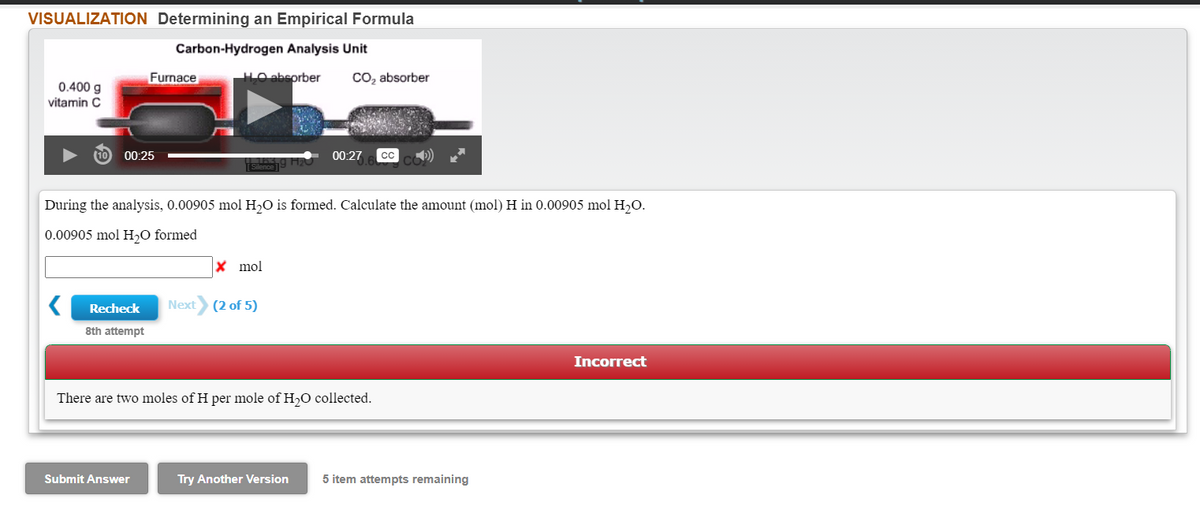
Transcribed Image Text:VISUALIZATION Determining an Empirical Formula
Carbon-Hydrogen Analysis Unit
Furnace
LO abeorber
Co, absorber
0.400 g
vitamin C
00:25
00:27
During the analysis, 0.00905 mol H,O is formed. Calculate the amount (mol) H in 0.00905 mol H,O.
0.00905 mol H,O formed
X mol
Recheck
Next
(2 of 5)
8th attempt
Incorrect
There are two moles of H per mole of H,O collected.
Submit Answer
Try Another Version
5 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning