Show how these elements combine into octet-rule ionic compounds, as in the first example. First, draw atomic Lewis structures for the atoms and their O.R. ions. Show their charges, and use them to find the unit formula, and the name. Then draw atomic Lewis structures for all atoms in the formula unit together, show with arrows how alle s are transferred to make the ons, and draw the Lewis structure of the ionic compound. Unit formula Atoms O.R. ions Name Transfer e s with arrows Lewis Structure 1е Li. N. LizN le 30 Lithium Li. + •N. Li :N: 30 Li. Li Li Li :N nitride le Li : Pb and K and H
Show how these elements combine into octet-rule ionic compounds, as in the first example. First, draw atomic Lewis structures for the atoms and their O.R. ions. Show their charges, and use them to find the unit formula, and the name. Then draw atomic Lewis structures for all atoms in the formula unit together, show with arrows how alle s are transferred to make the ons, and draw the Lewis structure of the ionic compound. Unit formula Atoms O.R. ions Name Transfer e s with arrows Lewis Structure 1е Li. N. LizN le 30 Lithium Li. + •N. Li :N: 30 Li. Li Li Li :N nitride le Li : Pb and K and H
Chapter1: Lewis Structures
Section: Chapter Questions
Problem 52EQ
Related questions
Question
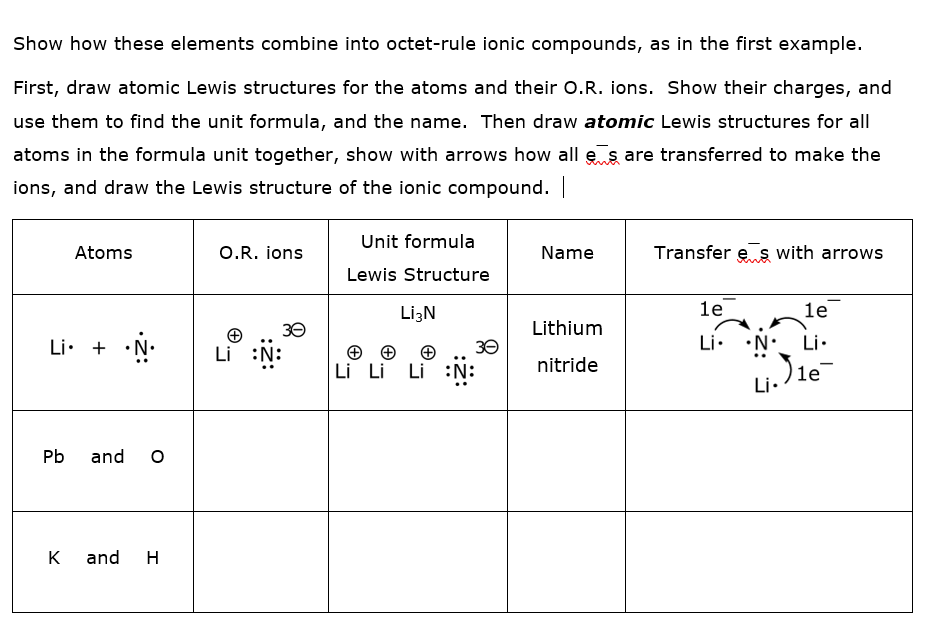
Transcribed Image Text:Show how these elements combine into octet-rule ionic compounds, as in the first example.
First, draw atomic Lewis structures for the atoms and their O.R. ions. Show their charges, and
use them to find the unit formula, and the name. Then draw atomic Lewis structures for all
atoms in the formula unit together, show with arrows how all e s are transferred to make the
ions, and draw the Lewis structure of the ionic compound.
Unit formula
Atoms
O.R. ions
Name
Transfer e s with arrows
Lewis Structure
LizN
le
1e
30
Lithium
Li. + •N•
Li.
•N• Li.
Li
30
Li Li Li :N:
nitride
)le
Li.
Pb
and
K
and
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning