
Concept explainers
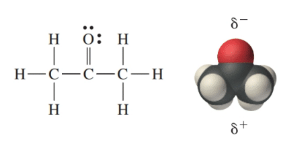
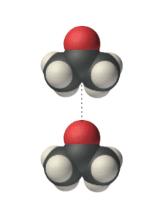
Acetone is a polar molecule; the oxygen end has a slightly negative charge (oxygen is more electronegative), whereas the carbon and hydrogen end has a slightly positive charge. In liquid acetone, the molecules are attracted to each other via these polar ends-the positive end of one molecule is attracted to the negative end of its neighbor-and therefore align as shown here:
Draw a Lewis structure and space-filling molecular image for
Trending nowThis is a popular solution!

Chapter 5 Solutions
Chemistry In Focus
- Consider the following compounds: CO2, SO2, KrF2, SO3, NF3, IF3, CF4, SF4, XeF4, PF5, TF5, and SCl6. These 12 compounds are all examples of different molecular structures. Draw the Lewis structures for each and predict the molecular structures. Predict the bond angles and the polarity of each. (A polar molecule has a net dipole moment, while a nonpolar molecule does not.) See Exercises 25 and 26 for the molecular structures based on the trigonal bipyramid and the octahedral geometries.arrow_forwardSuccessive substitution of F atoms for H atoms in the molecule NH3 produces the molecules NH2F, NHF2, and NF3. a. Draw Lewis structures for each of the four molecules. b. Using VSEPR theory, predict the geometry of each of the four molecules. c. Specify the polarity (polar or nonpolar) for each of the four molecules.arrow_forwardConsider the following compounds: CO2, SO2, KrF2, SO3, NF3, IF3, CF4, SF4, XeF4, PF5, IF5, and SCl6. These 12 compounds arc all examples of different molecular structures. Draw the Lewis structures for each and predict the molecular structure. Predict the bond angles and the polarity of each. (A polar molecule has a net dipole moment, while a nonpolar molecule docs not.) See Exercises 115 and 116 for the molecular structures based on the trigonal bipyramid and the octahedral geometries.arrow_forward
- The molecules BF3, CF4, CO2, PF5, and SF6 are all nonpolar, even though they all contain polar bonds. Why?arrow_forwardDraw a Lewis structure for each of the following molecules or ions. (a) CS2 (b) BF4 (c) HNO2 (where the bonding is in the order HONO) (d) OSCl2 (where S is the central atom)arrow_forwardBest Lewis Formula and Molecular Geometry A student writes the Lewis electron-dot formula for the carbonate anion, CO32, as a Does this Lewis formula obey the octet rule? Explain. What are the formal charges on the atoms? Try describing the bonding for this formula in valence bond terms. Do you have any difficulty doing this? b Does this Lewis formula give a reasonable description of the electron structure, or is there a better one? If there is a better Lewis formula, write it down and explain why it is better. c The same student writes the following resonance description for CO2: Is there something wrong with this description? (What would you predict as the geometries of these formulas?) d Is one or the other formula a better description? Could a value for the dipole moment help you decide? e Can you write a Lewis formula that gives an even better description of CO2? Explain your answer.arrow_forward
- Acrolein is the starting material for certain plastics. (a) Which bonds in the molecule are polar and which are nonpolar? (b) Which is the most polar bond in the molecule? Which atom is the partial negative end of this bond?arrow_forwardPredict die molecular structure and bond angles for each molecule or ion in Exercises 88 and 94. a. POCl3, SO42, XeO4, PO43, ClO4 b. NF3, SO32, PO33, ClO3 c.ClO2, SCl2, PCl2 d. Considering your answers to parts a, b, and c. what conclusions can you draw concerning the structures of species containing the same number of atoms and the same number of valence electrons? (O3), sulfur dioxide, and sulfur trioxide.arrow_forwardSuccessive substitution of F atoms for H atoms in the molecule CH4 produces the molecules CH3F, CH2F2, CHF3, and CF4. a. Draw Lewis structures for each of the five molecules. b. Using VSEPR theory, predict the geometry of each of the five molecules. c. Specify the polarity (polar or nonpolar) for each of the five molecules.arrow_forward
- Which molecule has the most polar bond: N2, BrF, or ClF? Use an arrow to show the direction of polarity in each bond.arrow_forwardConsider the following molecules: SiH4, PH3, H2S. In each case, a central atom is surrounded by four electron pairs. In which of these molecules would you expect the bond angle to be less than 109.5? Explain your reasoning.arrow_forwardIndicate which of the following molecules are polar. Draw the molecular structure of each polar molecule, including the arrows that indicate the bond dipoles and the molecular dipole moment. (a) HCN (b) I2 (c) NOarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





