• Part B Now consider the reaction A + 2B C for which in the initial mixture Q. = 387 %3D Is the reaction at equilibrium? If not, in which direction will it proceed to reach equilibrium? > View Available Hint(s) O Thereaction is already at equilibrium. The reaction will proceed in reverse to form reactants The reaction will proceed forward to form products Submit
• Part B Now consider the reaction A + 2B C for which in the initial mixture Q. = 387 %3D Is the reaction at equilibrium? If not, in which direction will it proceed to reach equilibrium? > View Available Hint(s) O Thereaction is already at equilibrium. The reaction will proceed in reverse to form reactants The reaction will proceed forward to form products Submit
Chapter13: Chemical Equilibrium
Section: Chapter Questions
Problem 10RQ: The only stress (change) that also changes the value of K is a change in temperature. For an...
Related questions
Question
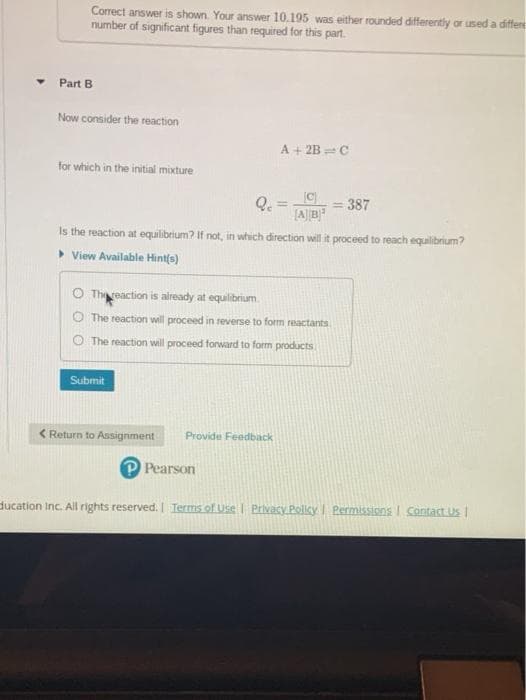
Transcribed Image Text:Correct answer is shown. Your answer 10.195 was either rounded differently or used a differe
number of significant figures than required for this part.
Part B
Now consider the reaction
A + 2B = C
for which in the initial mixture
= 387
%3D
%3D
Is the reaction at equilibrium? If not, in which direction will it proceed to reach equilibrium?
> View Available Hintfs)
O Threaction is already at equilibrium.
O The reaction will proceed in reverse to form reactants
O The reaction will proceed forward to form products
Submit
< Return to Assignment
Provide Feedback
Pearson
ducation Inc. All rights reserved. I Terms of Use | Privacy Polcy | Permissions I Contact Us
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning