- Part B Rank the following elements by electron affinity, from most positive to most negative EA value. Rank from most positive to most negative. To rank items as equivalent, overlap them. • View Available Hint(s) Reset Help iodine bismuth krypton tellurium cesium
- Part B Rank the following elements by electron affinity, from most positive to most negative EA value. Rank from most positive to most negative. To rank items as equivalent, overlap them. • View Available Hint(s) Reset Help iodine bismuth krypton tellurium cesium
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 12ALQ
Related questions
Question
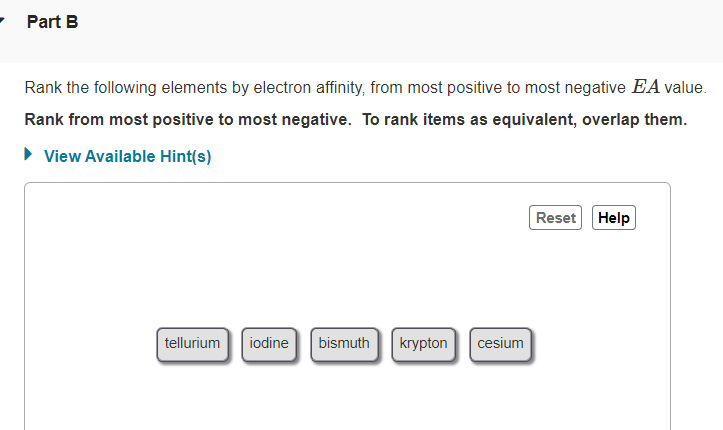
Transcribed Image Text:Part B
Rank the following elements by electron affinity, from most positive to most negative EA value.
Rank from most positive to most negative. To rank items as equivalent, overlap them.
• View Available Hint(s)
Reset Help
iodine
krypton
tellurium
bismuth
cesium

Transcribed Image Text:Part A
For the elements with the electron affinities given in the table in the introduction, which element is most likely to accept an
electron?
• View Available Hint(s)
OS
O Ca
O N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co