Part D Based on your answer to Part B or C, what is the average rate of change of H2 ? Remember that reactant concentrations decrease over time. Express your answer to three decimal places and include the appropriate units. • View Available Hint(s)
Part D Based on your answer to Part B or C, what is the average rate of change of H2 ? Remember that reactant concentrations decrease over time. Express your answer to three decimal places and include the appropriate units. • View Available Hint(s)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter13: Chemical Kinetics
Section: Chapter Questions
Problem 13.5QE: Explain why half-lives are not normally used to describe reactions other than first order.
Related questions
Question
100%
Please answer the question
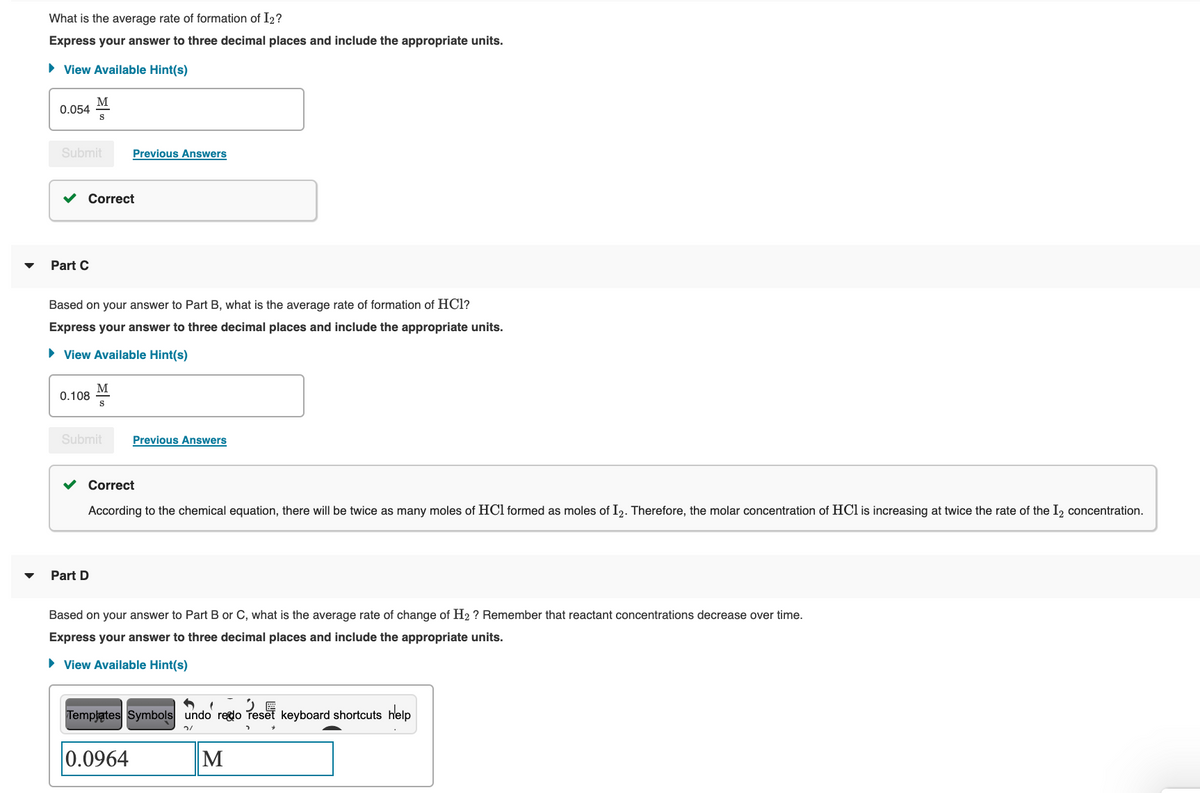
Transcribed Image Text:What is the average rate of formation of I2?
Express your answer to three decimal places and include the appropriate units.
• View Available Hint(s)
M
0.054
Submit
Previous Answers
v Correct
Part C
Based on your answer to Part B, what is the average rate of formation of HCl?
Express your answer to three decimal places and include the appropriate units.
• View Available Hint(s)
M
0.108
Submit
Previous Answers
v Correct
According to the chemical equation, there will be twice as many moles of HCl formed as moles of I2. Therefore, the molar concentration of HCl is increasing at twice the rate of the I, concentration.
Part D
Based on your answer to Part B or C, what is the average rate of change of H2 ? Remember that reactant concentrations decrease over time.
Express your answer to three decimal places and include the appropriate units.
• View Available Hint(s)
Templątes Symbols undo redo Teset keyboard shortcuts help
0.0964
M
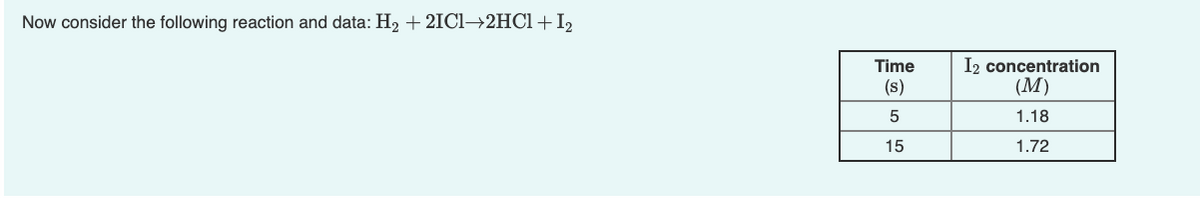
Transcribed Image Text:Now consider the following reaction and data: H2+ 2IC1→2HC1+I2
I2 concentration
(M)
Time
(s)
1.18
15
1.72
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning