CHEM 1111 Molar Mass of Butane Data Sheet These tables and all work for calculations are to be included in the laboratory notebook. UNITS TRIAL 1 TRIAL 2 TRIAL 3 Final Mass of Lighter 16.134 15.321 15.142 Initial Mass of Lighter 166.304 15.907 15.321 Mass of Butane 0.17 0.586 0.179 Volume of Gas Collected 81.5 80.5 mL 80.5 Air Temperature Kelvins 295.15 Water Temperature 22.0 Vapor Pressure of H20 Barometric Pressure cm 74.8 Calculations 1. Use Equation 2 to determine the partial pressure of butane in the graduated cylinder for each trial.
CHEM 1111 Molar Mass of Butane Data Sheet These tables and all work for calculations are to be included in the laboratory notebook. UNITS TRIAL 1 TRIAL 2 TRIAL 3 Final Mass of Lighter 16.134 15.321 15.142 Initial Mass of Lighter 166.304 15.907 15.321 Mass of Butane 0.17 0.586 0.179 Volume of Gas Collected 81.5 80.5 mL 80.5 Air Temperature Kelvins 295.15 Water Temperature 22.0 Vapor Pressure of H20 Barometric Pressure cm 74.8 Calculations 1. Use Equation 2 to determine the partial pressure of butane in the graduated cylinder for each trial.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 54E: DDT (molar mass = 354.49 g/mol) was a widely used insecticide that was banned from use in the United...
Related questions
Question
I have the data, I only need the calculations of the last page.
Transcribed Image Text:CHEM 1111
Molar Mass of Butane
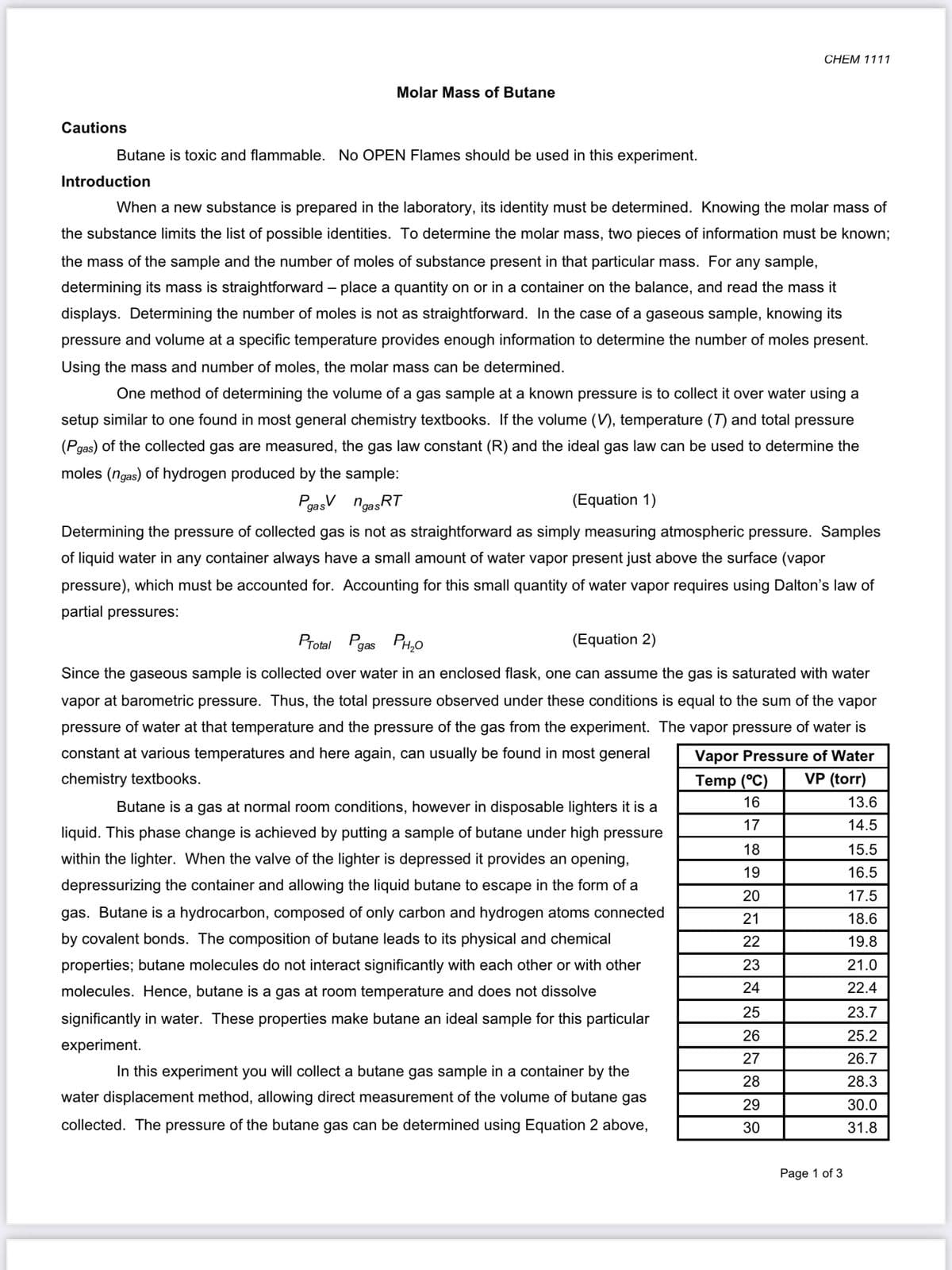
Cautions
Butane is toxic and flammable. No OPEN Flames should be used in this experiment.
Introduction
When a new substance is prepared in the laboratory, its identity must be determined. Knowing the molar mass of
the substance limits the list of possible identities. To determine the molar mass, two pieces of information must be known;
the mass of the sample and the number of moles of substance present in that particular mass. For any sample,
determining its mass is straightforward – place a quantity on or in a container on the balance, and read the mass it
displays. Determining the number of moles is not as straightforward. In the case of a gaseous sample, knowing its
pressure and volume at a specific temperature provides enough information to determine the number of moles present.
Using the mass and number of moles, the molar mass can be determined.
One method of determining the volume of a gas sample at a known pressure is to collect it over water using a
setup similar to one found in most general chemistry textbooks. If the volume (V), temperature (T) and total pressure
(Pgas) of the collected gas are measured, the gas law constant (R) and the ideal gas law can be used to determine the
moles (ngas) of hydrogen produced by the sample:
PgasV n
ngasRT
(Equation 1)
Determining the pressure of collected gas is not as straightforward as simply measuring atmospheric pressure. Samples
of liquid water in any container always have a small amount of water vapor present just above the surface (vapor
pressure), which must be accounted for. Accounting for this small quantity of water vapor requires using Dalton's law of
partial pressures:
Protal Pgas
PH,0
(Equation 2)
Since the gaseous sample is collected over water in an enclosed flask, one can assume the gas is saturated with water
vapor at barometric pressure. Thus, the total pressure observed under these conditions is equal to the sum of the vapor
pressure of water at that temperature and the pressure of the gas from the experiment. The vapor pressure of water is
constant at various temperatures and here again, can usually be found in most general
Vapor Pressure of Water
chemistry textbooks.
Temp (°C)
VP (torr)
16
13.6
Butane is a gas at normal room conditions, however in disposable lighters it is a
17
14.5
liquid. This phase change is achieved by putting a sample of butane under high pressure
18
15.5
within the lighter. When the valve of the lighter is depressed it provides an opening,
19
16.5
depressurizing the container and allowing the liquid butane to escape in the form of a
20
17.5
gas. Butane is a hydrocarbon, composed of only carbon and hydrogen atoms connected
21
18.6
by covalent bonds. The composition of butane leads to its physical and chemical
22
19.8
properties; butane molecules do not interact significantly with each other or with other
23
21.0
molecules. Hence, butane is a gas at room temperature and does not dissolve
24
22.4
25
23.7
significantly in water. These properties make butane an ideal sample for this particular
26
25.2
experiment.
27
26.7
In this experiment you will collect a butane gas sample in a container by the
28
28.3
water displacement method, allowing direct measurement of the volume of butane gas
29
30.0
collected. The pressure of the butane gas can be determined using Equation 2 above,
30
31.8
Page 1 of 3
Transcribed Image Text:CHEM 1111
Molar Mass of Butane
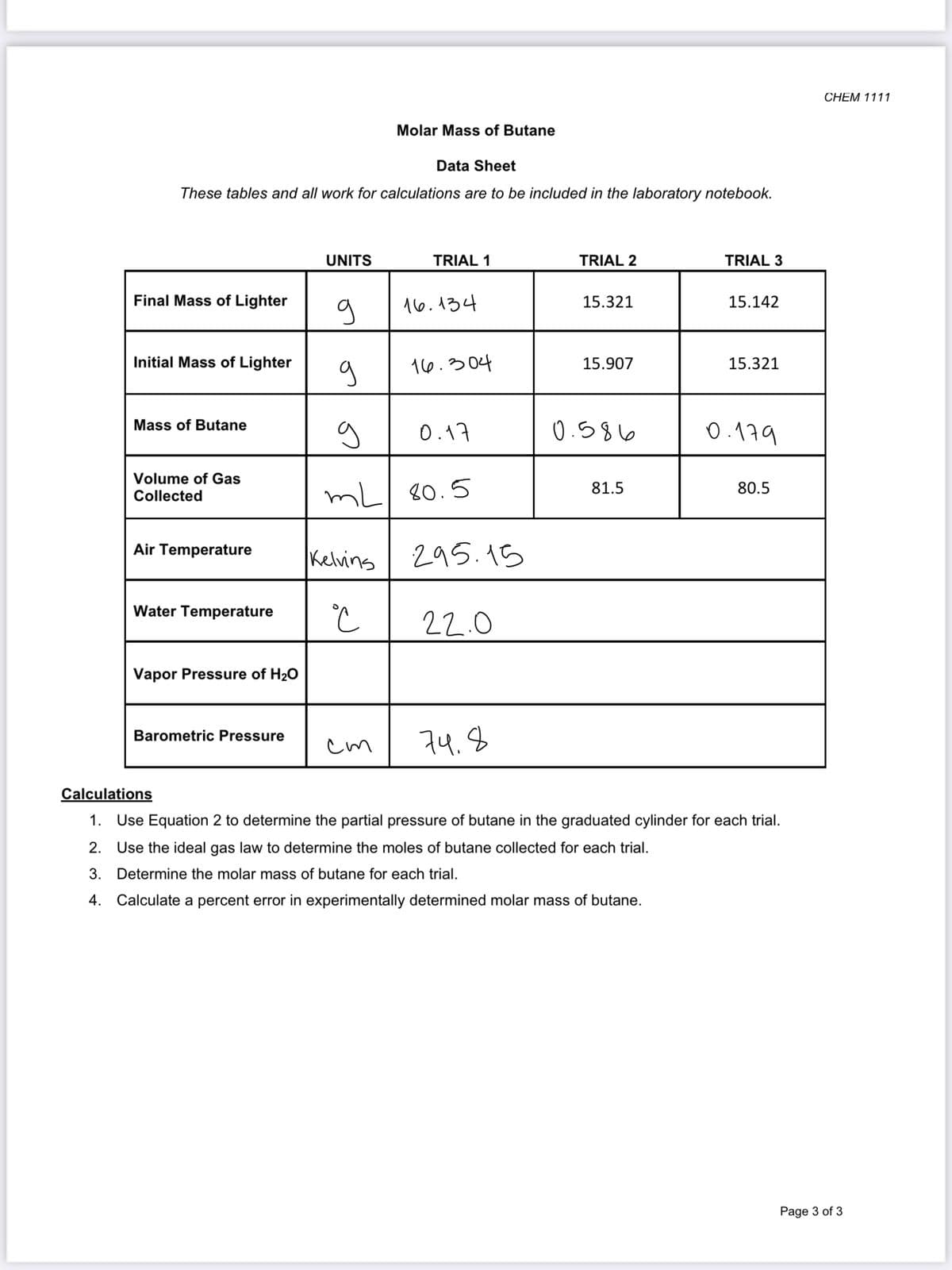
Data Sheet
These tables and all work for calculations are to be included in the laboratory notebook.
UNITS
TRIAL 1
TRIAL 2
TRIAL 3
Final Mass of Lighter
16.134
15.321
15.142
Initial Mass of Lighter
16.304
15.907
15.321
Mass of Butane
0.17
0.586
0.179
Volume of Gas
81.5
80.5
mL 80.5
Collected
Air Temperature
Kelvins
295.15
Water Temperature
22.0
Vapor Pressure of H20
Barometric Pressure
cm
74.8
Calculations
1. Use Equation 2 to determine the partial pressure of butane in the graduated cylinder for each trial.
2. Use the ideal gas law to determine the moles of butane collected for each trial.
3.
Determine the molar mass of butane for each trial.
4.
Calculate a percent error in experimentally determined molar mass of butane.
Page 3 of 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning