Phosphoric acid is made industrially by the reaction of fluorapatite, Cas(PO4);F, in phosphate rock with sulfuric acid: Ca5(PO4);F(s) + 5 H,SO4(aq) + 10 H,O(€) → 3 H;PO4(aq) + 5 (CaSO4-2H,O)(s) + HF(aq) What volume of 6.3 M phosphoric acid is generated by the reaction of 2.2 metric tons (2200 kg) of fluorapatite?
Phosphoric acid is made industrially by the reaction of fluorapatite, Cas(PO4);F, in phosphate rock with sulfuric acid: Ca5(PO4);F(s) + 5 H,SO4(aq) + 10 H,O(€) → 3 H;PO4(aq) + 5 (CaSO4-2H,O)(s) + HF(aq) What volume of 6.3 M phosphoric acid is generated by the reaction of 2.2 metric tons (2200 kg) of fluorapatite?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.93PAE: 93 The complete combustion of octane can be used as a model for the burning of gasoline:...
Related questions
Question
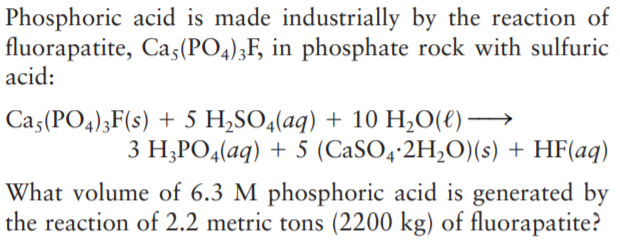
Transcribed Image Text:Phosphoric acid is made industrially by the reaction of
fluorapatite, Cas(PO4);F, in phosphate rock with sulfuric
acid:
Ca5(PO4);F(s) + 5 H,SO4(aq) + 10 H,O(€) →
3 H;PO4(aq) + 5 (CaSO4-2H,O)(s) + HF(aq)
What volume of 6.3 M phosphoric acid is generated by
the reaction of 2.2 metric tons (2200 kg) of fluorapatite?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning