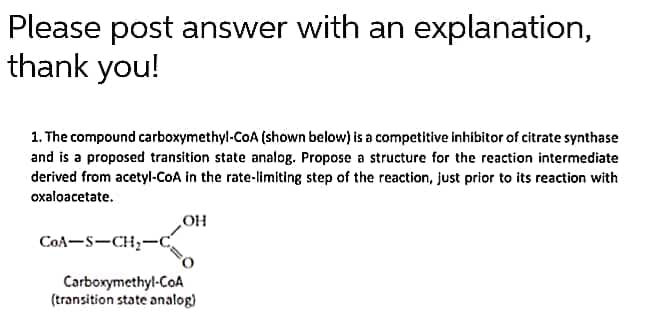
Please post answer with an explanation, thank you! 1. The compound carboxymethyl-CoA (shown below) is a competitive inhibitor of citrate synthase and is a proposed transition state analog. Propose a structure for the reaction intermediate derived from acetyl-COA in the rate-limiting step of the reaction, just prior to its reaction with oxaloacetate. но CoA-S-CH,-c Carboxymethyl-CoA (transition state analog)
Q: A. Which one of the following is an example of affinity chromatography? Select all that apply. a) Ho...
A: The separation technique called affinity chromatography is based on the principle of particular bind...
Q: Derive a mathematical equation that calculates the speed at which DNA molecules move when placed ins...
A: Electrophoresis is a process where DNA is separated on the basis of there molecular size. the term E...
Q: Ex: If the radiation intensity of the surface is 10& tissue for treatment half of the intensity is a...
A: Given Values: Radiation intensity at the surface = 10 The intensity absorbed by the 3 cm tissue = 5
Q: Which of the following statements best describe(s) the mechanism by which correct protein folding ta...
A: Chaperonin GroEL/GroES is a molecular chaperone that facilitates folding of nascent or denatured pro...
Q: Need both parts answered
A: Protein is a biomolecule that is the key unit for the structural formation of cell and cell organell...
Q: Please help
A: This crossword is based on the "genetics" that is the study of genes and inheritance patterns exhibi...
Q: Is this the correct answer of R and T in the blanks or are they switched?
A: Proteins are composed of twenty standard amino acids attached together via peptide bonds. These twen...
Q: 35 Which statement about BPG and hemoglobin is FALSE? Question 35 options: fetal hemoglobin h...
A: Red blood cells carry hemoglobin, which is a protein. Hemoglobin absorbs oxygen from the lungs and t...
Q: How would your body look without epithelial tissue. What would be missing on the outside and on the ...
A: The answer of the following question is given below
Q: Explain in a more clinical detail the mode of actions of the following on the human skin cell or tis...
A: “Since you have asked multiple questions, we will solve the first question for you. If you want any ...
Q: Explain your answer in 1-3 sentences only. FOLLOW INSTRUCTION! 1. Lectins are a. sugars specific to ...
A: Lectins are a type of biomolecules found in cells. Lectins are found in lentils, tomatoes, beans, pe...
Q: What are different kind of forces present in primary , secondary and Tertiary structure of protein ....
A: Amino acids are the basic structural and functional units of proteins. They are the monomeric units ...
Q: Explain how temperature and pH affect enzyme activity from a biochemical standpoint. What are the ch...
A: Enzymes are the biochemical catalysts that fasten the rate of the reaction in our body. In the absen...
Q: Kindly answer numbers 12, 13, and 14 12. This protein that is involve in immune system responses is...
A: Note - Hi ! Thank you for the question. We are authorized to answer one question at a time. Since yo...
Q: Why is gluconeogenesis important in the Cori cycle?
A: The metabolic mechanism gluconeogenesis produces glucose from non-carbohydrate carbon sources. Plant...
Q: 1. Lectins are ______. (Explain your answer in 1-3 sentences.) A. sugars specific to proteins B. pro...
A: Since there are multiple questions and they are not interlinked, as per our company guidelines only ...
Q: 1. Describe the protein composition of gluten
A: “Since you have asked multiple question, we will solve the first question for you. If youwant any sp...
Q: Affinity purification: Assemble the following components to set up affinity chromatography to purify...
A: Affinity chromatography: It is a chromatographic technique for the separation of the compound on th...
Q: Please answer fast First dar a diacylphosphogylerol in which both acyl-groups resulte from condensa...
A: omega 3 18:1 fatty acid has 18 C-atoms with one double bond in between C3 and C4 carbon atom from om...
Q: Tabulate the different protein precipitation methods by completing the table below.
A: There are different methods of protein precipitation like organic solvent precipitation, ammonium su...
Q: . What is nanotechnology?
A: “Since you have asked multiple question, we will solve the first question for you. If you want any s...
Q: Explain the importance of glycogen loading related to athletes and coaches
A: Glycogen is a polysaccharide of glucose, it is multibranched made up of many connected glucose. It ...
Q: 1. What is the effect of hemolysis in the analysis of serum LPS? 2. Differentiate Lipase activity ...
A: Lipopolysaccharides (LPS) are huge molecules that are found in the outer membrane of Gram-negative b...
Q: Give one or two product of Milk and answer the following queston: look at the labels of the differen...
A: "Since you have posted a question with multiple sub-parts, we will solve first three sub parts for y...
Q: A protein has an isoelectric point at 6. It is soluble at what pH level? A. 6 B. 10 C. 8 D. 4
A: The isoelectric point of a protein is the pH at which the net charge of the protein is zero. The cha...
Q: With the aid of a diagram, explain the principles of gel filtration chromatography.
A: Gel filtration chromatography: Chromatography is an analytical technique where the components of a ...
Q: 1. CONCEPTS: DIRECT DESCRIPTIONS: 1.Enzyme infl...
A: Hi.Thank you for the question. As per the honor code, we are allowed to answer three sub-parts at a ...
Q: Multiple answers: Multiple answers are accepted for this question Select one or more answers and sub...
A: Enzymes are usually made of protein molecules which catalyze biochemical reactions by lowering the a...
Q: UDP , gemcitabine was shown to undergo two successive phosphorylations at . The product, gemcitabine...
A: GEMCITABINE is a chemotherapy medication, used to treat cancer such as breast cancer, testicular can...
Q: Which characteristic is truc of protcins? O Proteins have up to four levels of structure. O Proteins...
A: Proteins are organic compounds which consists of amino acids, which are essential for carrying out b...
Q: Differentiate the structures and functions of prokaryotic and eukaryotic cell. Provide at-least 3 ex...
A: Cell is the basic structural and functional unit of life.
Q: 5) A stock solution of glucose was prepared by dissolving 500 mg of glucose in distilled water to a ...
A: The Nelson-Somogyi method/assay is a method that is used to measure reducing sugars quantitatively. ...
Q: Choose one example of a carbohydrates with a chiral carbon and draw its Fisher's and Haworth's struc...
A: Carbohydrates are classified as monosaccharides, oligosaccharides, and polysaccharides based on the ...
Q: A polysaccharide conposed of entirely fructose units is inulin. Which carbohydrates test should be u...
A: Inulin is a homopolysaccharide, which is composed of fructose units. Qualitative tests for carbohydr...
Q: Salivary amylase is used to hydrolyzed α(1à4) glycosidic bonds in carbohydrates. True False
A: Enzymes are proteinaceous in nature. Almost all enzymes are proteins, there are some nucleic acids t...
Q: Is this the correct answer of finding what the charge is?
A: Amino acids are the smallest units that make up the peptides or proteins inside the cell. Twenty sta...
Q: iscuss shortly the main differences among the different types of carbohydrates (monosaccharide, disa...
A: Carbohydrates: Carbohydrates are sugars that provide the body with energy and it is composed of org...
Q: Mutarotation affects the reducing property of carbohydrates. True False
A: Mutarotation: It is a spontaneous change that takes place in the optical rotation of alpha and beta...
Q: Please answer questions 1 and 2 directly. 1. Discuss what is isoelectric precipitation/point? 2. Dif...
A: The given diagram shows isolation by caesin by isoelectric precipitation
Q: Please help a bit confused
A: STR is defined as the Short tandem repeats, also known as microsatellites or simple sequence repeats...
Q: Please directly answer the theoretical background (positive color reactions and what is being detect...
A: All the given tests are qualitative tests for detection of protein. The tests are as follows: Biure...
Q: True or false? Under anaerobic conditions, the pyruvate dehydrogenase complex is responsible for th...
A: Pyruvate dehydrogenase complex (PDHC) of three enzymes which converts pyruvate into acetyl CoA by de...
Q: 13
A: 20:2 Delta 4,9 fatty acid It means fatty acid with 20 carbons and 2 double bonds at 4th , 9th carbon...
Q: What is glycemic index (GI)? Which food in the Philippine market has significant GI and list them wi...
A: GI is also known as Glycemic Index
Q: Before Lab: Compare the structures below to the structure of the substrate. Which do you think would...
A: Alkaline phosphatase (AP) is a group of enzymes which removes phosphate groups from the substrates. ...
Q: What are the similarities and difference between amylopectin and glycogen? Give at least 2 Similarit...
A: Glycogen : Glucose polymer Amylopectin- One of the two constituent of starch, while the other being ...
Q: . Describe the mechanism of action of the denaturing agents used -a. Detergents - b. Strong acid -c....
A: Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for yo...
Q: TRUE OR FALSE a) The long solenoid structure of the chromatin material binds to a protein scaffold ...
A: The DNA of a cell and its related proteins make up chromatin. In eukaryotic chromatin, histone prote...
Q: Non-additive genetic factors make children less resemble their parents. true or false (reason in y...
A: Heredity is the continuity of features from one generation to another and it can be defined as the r...
Q: Does celluloid have something to do with cellulose?
A: Cellulose is a polysaccharide, composed of linear chain of thousands of β(1→4) linked D-glucose unit...
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- Need help, please. Given that the enzyme pyruvate carboxylase converts Pyruvate into Oxaloacetate and this is an anapleurotic reaction. In the liver, what is most of the OAA converted into under these conditions? Please explain. 1. When blood glucose levels are low and the energy charge in the cell is high. 2. When mitochondrial acetyl-CoA levels are high and the energy charge is low.Please state if the statements are true or false.1. When fructose forms its Haworth projection, it usually assumes a pyranose structure2. The conversion of 1 mole aplha-ketoglutarate to 1 mole succinyl CoA produces 2 moles of carbon dioxideInstructions. Given each set of information which may include common name(s) and the reaction catalyzed, you are required to identify the main class of the specific enzyme described. Name: citryl-CoA synthetase Reaction: ATP + citrate + CoA = ADP + phosphate + (3S)-citryl-CoA Name: D-xylulose reductase Reaction: xylitol + NAD+ = D-xylulose + NADH + H+ Name: cellobiose phosphorylase Reaction: cellobiose phosphate = α-D-glucose 1-phosphate + D-glucose Name: carbonic anhydrase Reaction: H2CO3 = CO2 + H2O Other info: The enzyme catalyzes the reversible hydration of gaseous CO2 to carbonic acid, which dissociates to give hydrogencarbonate above neutral pH. Name: pantoate activating enzyme Reaction: ATP + (R)-pantoate = AMP + diphosphate + (R)-pantothenate.
- Please please help me with these, thank you so muchshow the de novo synthesis of capric acid and which pathways produce NADPH? How is acetyl coA carboxylase being regulated?Consider docosanoic acid C12H43CO2H a. Label the alpha and beta Carbons. Show the beta-oxidation in an EXPANDED structure. b. Draw each acyl CoA derived from this fatty acid. c. How many acetyl Co A molecules are formed by complete beta-oxidation? d. How many cycles of beta-oxidation are needed for complete oxidation? e. How many molecules of ATP are formed from the complete catabolism of this fatty acid? Show the complete computation. f. How many moles of ATP per gram of fatty acid is formed from the complete catabolism of the given fatty acid? g. What is the molar mass of the given fatty acid? Solution: Show here the complete computations, [from a to e]For myristic acid, C 13H 27CO 2H: (a) How many molecules of acetyl CoA are formed from complete β-oxidation? (b) How many cycles of β-oxidation are needed for complete oxidation?
- Extending the Mechanism of Methylmalonyl-CoA Mutase to Similar Reactions Based on the mechanism for the methylmalonyl-CoA mutase (see problem 14), write reasonable mechanisms for the following reactions shown.Understanding the Mechanism of the -Ketoglutarate Dehydrogenase Reaction tshe first step of the -Ketoglutarate dehydrogenase reaction involves decarboxylation of the substrate and leaves a covalent TPP intermediate. Write a reasonable mechanism for this reaction.Instructions. Given each set of information which may include common name(s) and the reaction catalyzed, you are required to identify the main class of the specific enzyme described. _____________________ Name: alkaline phosphatase Reaction: a phosphate monoester + H2O = an alcohol + phosphate _____________________ Reaction: L-threonine = D-threonine. Other information: Inverts both chiral centers, a racemase. _____________________ Name: glycine-N-acylase Reaction: acyl-COA + glycine = CoA + N-acylglycine _____________________ Name: lysine decarboxylase Reaction: L-lysine = cadaverine + CO2 _____________________ Name: methanol dehydrogenase Reaction: methanol + NAD+ = formaldehyde + NADH + H+ _____________________ Name: citryl-CoA synthetase Reaction: ATP + citrate + CoA = ADP + phosphate + (3S)-citryl-CoA _____________________ Name: D-xylulose reductase Reaction: xylitol + NAD+ = D-xylulose + NADH + H+ _____________________ Name: cellobiose phosphorylase Reaction:…
- Instructions. Given each set of information which may include common name(s) and the reaction catalyzed, you are required to identify the main class of the specific enzyme described. _____________________ Name: alkaline phosphatase Reaction: a phosphate monoester + H2O = an alcohol + phosphate _____________________ Reaction: L-threonine = D-threonine. Other information: Inverts both chiral centers, a racemase. _____________________ Name: glycine-N-acylase Reaction: acyl-COA + glycine = CoA + N-acylglycine _____________________ Name: lysine decarboxylase Reaction: L-lysine = cadaverine + CO2 _____________________ Name: methanol dehydrogenase Reaction: methanol + NAD+ = formaldehyde + NADH + H+CHOOSE THE CORRECT LETTER 1.What are the B-oxidation products of lauric acid (C12H2402), a major component of coconut oil?A.6 acetyl-CoA, 6 NADH, 6 FADH2B. 6 acetyl-CoA, 5 NADH, 5 FADH2C. 5 acetyl-CoA, 6 NADH, 6 FADH2D. 5 acetyl-CoA, 5 NADH, 5 FADH2 2.What is the mechanism of ATP synthesis in glycolysis?A. substrate level phosphorylationB. reductionC. oxidationD. oxidative phosphorylation 3.Which of the following enzymes need ATP as a substrate in the reaction?A.phosphoglycerate kinaseB. phosphofructokinaseC. pyruvate kinaseD.glyceraldehyde-3-phosphate dehydrogenaseConsidering the fatty acids: (a) Arachidic acid (C20H40O2); molar mass = 312.5 g/mol) (b) Palmitoleic acid (C16H30O2); molar mass = 256.4 g/mol). How many cycles of β -oxidation are needed for complete oxidation? How many molecules of acetyl CoA are formed from its complete catabolism? How can you calculate the number of molecules (moles) of ATP formed (net) by the complete catabolism of each fatty acid? and the number of moles of ATP formed per gram of each fatty acid metabolized??

