possible and show complete solution. Round your final answer to 4 decimal places and box / highlight all final answers. and all values must include proper units with proper conversion if needed in your solution. 5. Calculate the boiling point of a solution that contains 1.5 grams of glycerin in 30 grams of water. #use the other pictures as reference or basis as needed in your solution
possible and show complete solution. Round your final answer to 4 decimal places and box / highlight all final answers. and all values must include proper units with proper conversion if needed in your solution. 5. Calculate the boiling point of a solution that contains 1.5 grams of glycerin in 30 grams of water. #use the other pictures as reference or basis as needed in your solution
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter7: Sollutions And Colloids
Section: Chapter Questions
Problem 7.122E
Related questions
Question
100%
kindly help me with this problem thank you
Instruction: solve as neatly as possible and show complete solution. Round your final answer to 4 decimal places and box / highlight all final answers. and all values must include proper units with proper conversion if needed in your solution.
5. Calculate the boiling point of a solution that contains 1.5 grams of glycerin in 30 grams of water.
#use the other pictures as reference or basis as needed in your solution
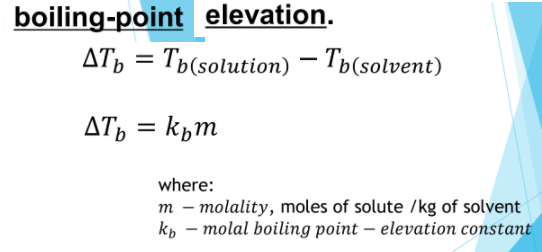
Transcribed Image Text:boiling-point elevation.
дть 3D Ть(solution) — Ты(solvent)
AT, = kpm
where:
m – molality, moles of solute /kg of solvent
kp – molal boiling point – elevation constant
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning