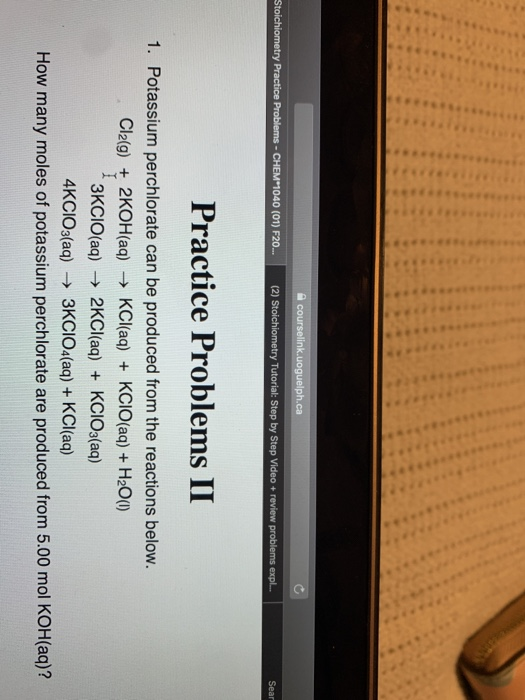
Practice Probler tassium perchlorate can be produced from the Cl2(g) + 2KOH(aq) → KCl(aq) + KCIO(a 3KCIO(aq) → 2KCI(aq) + KCIO 4KCIO3(aq) → 3KCIO4(aq) + KCI many moles of potassium perchlorate are p
Q: ▶ Practice Exercise 2When a 2.00-g strip of zinc metal is placed in an aqueoussolution containing…
A: Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the question and…
Q: Calculate the moles of 02 that will be prepared through the decomposition (see balanced reaction) of…
A: Given: Number of moles of potassium chlorate are 4.7 moles. Introduction: Write the balanced…
Q: Practice Question •What is being oxidized in this reaction? - NADH + ADP + Phosphate + O2 + H* →…
A: Loss of hydrogen or gain of Oxygen is oxidation. Loss of Oxygen or gain of hydrogen is reduction.…
Q: Question 1 Objective Knowledge Check Decide whether a chemical reaction happens in either of the…
A:
Q: Practice assignment: Stoichionmetry On a separate sheet of paper, show the setup and answer for each…
A:
Q: Practice Problem BUILD Using the chemical species A2, B, and AB, write a balanced equation for a…
A: The balanced equation for a combination reaction has to be given.
Q: Practice Exrercise The reaction between aluminum and iron(III) oxide can generate temperatures…
A: Given Mass of Al = 124 gMass of Fe2O3 = 601 gReaction: 2Al + Fe2O3 → Al2O3 + 2Fe To calculate: The…
Q: ACTIVITY 5.2 Instruction: Solve the following problems. 1. Assume that 10 grams of sodium and 5…
A: Given,
Q: Problem #5: Suppose 316.0 g aluminum sulfide reacts with 493.0 g of water. What mass of the excess…
A: Balance equation- Al2S3+ 6H2O-2Al(OH)3+3H2S 316 gm Al2S3 reacts with 493gm water Molecular mass of…
Q: Check my work Enter your answer in the provided box. Consider the combustion of butane (C,H10):…
A:
Q: PRACTICE EXAMPLE A: potassium chlorate? How many moles of O₂ are produced from the decomposition of…
A: The relationship between mass and number of moles is: Moles=MassMolar mass Known: Molar mass of…
Q: Practice Problem ATTEMPT Identify each of the following as a combination, decomposition, or…
A: The given reactions has to be identified as combination, decomposition or combustion reaction.
Q: One ingredient in some antacids is Al(OH)3 which reacts with stomach acid, HCl . Complete and…
A: Neutralization reaction: The hydrogen ion of acid reacts with the hydroxide ion of a base to form…
Q: Calculate the moles, to the nearest .01 mol, of O2 that will be prepared through the decomposition…
A: Recall the decomposition reaction of potassium chlorate : 2 KClO3 -------> 2 KCl + 3 O2 8.3…
Q: Practice Exercise 1The unbalanced equation for the reaction between methaneand bromine is__ CH41g2 +…
A: The reaction given is CH4 (g) + Br2 (l) ------> CBr4 (s) + HBr (g)
Q: Activity Count each of the atoms, find the total number, and number for each element. Subscript: The…
A: Since , we answer only three answer up to 3 sub-parts, we will answer the first three, Please…
Q: Problem 11 K 11 of Review I Constants I Periodic Butane, C4H10, reacts with oxygen, O2, to form…
A: The combustion of butane takes place according to the following reaction
Q: Problem #4: Interpret reactions in terms of representative particles, then write balanced chemical…
A: 4. a) The carbon atom combined with hydrogen to form methane. The equation for the balanced chemical…
Q: Practice Exercise 1Sodium hydroxide reacts with carbon dioxide to form sodiumcarbonate and water:2…
A: Number of moles is equal to the ratio of mass to molar mass. Its formula is Number of…
Q: 2 010 <Chapter 06 Problem 10 K 10 of 19 Review I Constants I Periodic Table Under certain…
A: The given reaction is,
Q: PRACTICE EXERCISE Classify each of the following chemical reactions as a combination, decomposition,…
A: a. is an example of decomposition reaction as in this reaction single reactant (potassium nitrate)…
Q: Submit Answer 2. Pick a "for every" statement about copper and silver nitrate for this balanced…
A: The balanced chemical reaction is: 1 Cu + 2 AgNO3 → 2 Ag + 1 Cu(NO3)2 The coefficient of Cu is…
Q: Skill Practice: Using Mole-Mole Relationships C3H8 5 02 -> 3 CO2 4 H20 1. How many moles of O, are…
A: One mole of propane reacts with five moles of oxygen to produce three moles of carbon dioxide and…
Q: Exercise: Assume that 10g of K;PtCl, and 10g of NHare react, how many grams of the excess reactant…
A:
Q: All change 9. What is the mole ratio of calcium carbonate to carbon dioxide in the reaction between…
A: Given reaction is, 2HCl + CaCO3 → CaCl2 + CO2 + H2O
Q: Calculate the volume (in mL) of 0.797 M HNO3 needed to react completely with 5.76 g of NiCO3 in a…
A: Given that - Molarity of nitric acid HNO3 = 0.797 M Mass of Nickel (II) Carbonate NiCO3 = 5.76 g…
Q: QUESTION 25 Balance the following equation with smallest whole number coefficients. What is the…
A: butane combustion reaction Butane molecular formula C4H10 Butane combustion reaction produce CO2…
Q: PRACTICE Complete the following chemical equations that show synthesis reactions involving metals…
A: Given, 2 K (s) + I2 (g) Sr (s) + Br2 (l) 2 V (s) + 3 Cl2 (g) Required,…
Q: I Review I Constants I Periodic Table Sodium hydroxide reacts with carbon dioxide as follows:…
A: Given : mole of NaOH = 2.30 mole Mole of Carbon dioxide = 1.20 moles To find: Limiting…
Q: Activity I 1. How many moles are there in 24.0 grams of FeF3? 2. How many moles are there in 458…
A: Since you are posted with multiple questions. As per the Bartleby guidelines, I am answering the…
Q: Exercise 2 Directions: Solve the following problems and show the complete solution. You may use a…
A:
Q: Practice Exercise 1Which is the correct net ionic equation for the reaction ofaqueous ammonia with…
A:
Q: Extra Practice : Predict the products with their states of matter, indicate the type of reaction,…
A: Given,
Q: Experiment #6: Synthesis of Malachite Mass of CuSO4 • 5H2O: 3.100 +/- 0.001 _g (with error) 1.524…
A: A numerical problem based on theoretical yield, which is to be accomplished.
Q: ACTIVITY 3.4.3. Solved me! 2. Calculate the AH for the reaction: CS2 (1) + 2 02 (g) CO2 (g) + 2 S02…
A: Enthalpy is a property of the thermodynamic system. It is defined as the sum of the internal energy…
Q: Free response: Given that when 44.76 mg of a compound only containing C, O, and H is burned, results…
A: Empirical formula is a simplest integer ratio of the atoms present in the compound. First divide the…
Q: Balancing Equations Practice Balance each of the following chemical equations. Directions: You are…
A:
Q: Activity Series Practice Predict whether or not each of the following reactions will occur. Write…
A: Explanation : Chemical Reaction : The process that when one or more compound are change to their…
Q: Practice Exercise Balance the equation representing the reaction between iron(II) oxide, Fe,03, and…
A: In balanced reaction number of each atom in reactant side should be equal to number of each atom in…
Q: Exercise 6.3 Give the balanced equation for each of the following reactions.a. When solid ammonium…
A: a).
Q: Consider the following reactions: N205 + 2 RbHCO3→2 RBNO3 + 2CO2 + H20 RBCIO3 + 3HNO2 RBCI + 3HNO3…
A: We have to choose the redox reaction for the following given two reactions as follows in step 2:
Q: For which of the following reactions is AHm equal to AH of the product(s)?You do not need to look…
A: From given Each reaction is checked whether ∆H°rxn is equal to ∆H°f and explained according
Q: ,AICI, 2. Zn/HCI
A:
Q: 1. Phosphorous reacts with oxygen gas to produce diphosphorous 2.When calcium comes in contact…
A: 1. Phosphorous ( P )reacts with oxygen gas ( O2 ) to produce diphosphorous pentoxide ( P2O5 ) 4…
Q: 10. More practice. The products for the reaction below are hydrogen gas and aluminum chloride. Write…
A: Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: EXERCISES A. What mass of copper can be made by reducing 16g of copper oxide with hydrogen? Equation…
A: A) given reaction CuO + H2 --------> Cu +H2O Mass of CuO = 16g
Q: Silver sulfide (Ag2S) is the common tarnish on silver objects. What weight of silver sulfide can be…
A: The reaction taking place is given as, => Ag (s) + H2S (g) + O2 (g) → Ag2S (s) + H2O (l) Given:…
Q: Activity 4 Write balanced reaction equations, including symbols describing the physical state of the…
A: Note - Since you have posted a question with multiple sub-parts, we will solve the first three…
Q: Practice Exercise The reaction between aluminum and iron(III) oxide ca temperatures approaching…
A: Given datas ; 2 Al + Fe2O3 ---> Al2O3 + 2 Fe Mass of Al = 124 g Mass of Fe2O3 = 601 g (a.)…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- 50.00 cm3 of a 1.5784 mol.dm-3 solution of potassium hydroxide is transferred to an empty 700.00 cm3 volumetric flask. This flask is made up to the mark with distilled water and then shaken well. The concentration of the potassium hydroxide in this second flask is:Potassium dichromate has several industrial applications. To determine the purity of the salt that will be used in different industrial processes, a sample mass equal to 2.660 g was dissolved and quantitatively transferred to a 500.00 mL flask. An aliquot of 25.00 mL of this solution was treated with excess KI and the released iodine was titrated with 0.1000 mol L-1 sodium thiosulfate, spending 27.00 mL. Calculate the purity of the analyzed salt. Data:K = 39.10 O = 16.00 Cr = 52.00 I = 126.9 S = 32.07Using the percent purity calculations, determine the percent yield of synthesis of aspirin. Part I Synthesis of Aspirin Mass of salicylic acid used (g) 2.029g Volume of acetic anhydride used (mL) 5ml Mass of acetic anhydride used (vol. × 1.08 g/mL) 5.4g Mass of aspirin synthesized (g) 3.256g Part II Melting Temperature Data Melting temperature (°C) 133°C Part III Salicylic Acid Standard Stock Solution Initial mass of salicylic acid (g) 0.210g Moles of salicylic acid (mol) 0.0147 mol Initial molarity of salicylic acid (M) 0.724 M Part III Beer’s Law Data for Salicylic Acid Standard Solutions Trial Concentration (M) Absorbance Water (mL) 1 10 0.301 0 2 7.5 0.219 2.5 3 5.0 0.163 5.0 4 2.5 0.074 7.5 Best-fit line equation for the salicylic acid standards Test of the Purity of the Synthesized Aspirin Initial mass of aliquot of product (g)…
- Beaker 0.00200 M Fe(NO3)3, mL 0.00200 M NaSCN, mL total volume, mL 1 3.000 2.000 10.00 2 3.000 3.000 10.00 3 3.000 4.000 10.00 4 3.000 5.000 10.00 5 (blank) 3.000 0.000 10.00 In Solutions 1-4 you are adding successively larger volumes of 0.00200M SCN- to the Fe3+ solution and diluting to 10.00 ml. Calculate the final diluted molarity of SCN- in solution #1 Your answer should have 3 sig figs =Eugenol, the main component of clove oil, can be prepared by heating 2-allyl guaiacol (in the absence of oxygen) to temperatures >205 °C. In practice, however, it is most cost-effective to extract eugenol from cloves, its natural source. Given a weight percentage of eugenol in cloves at 15.0%, calculate the minimum mass of cloves one needs to obtain 8.00 g of eugenol as a product. Report your answer (as a mass in grams, without units) with three significant digits.1) Commercial fuming Sulphuric acid (Oleum-H2S2O6) is 99.9% solution. Please convert it into molarity.2) Find out the Volume (dm3) of product (gas) at RTP when 0.58 M, 150 mL NaOH (aq.) reacts with 350 mL, 0.25 NH4Cl.
- Gravimetric analysis of Fe3O4 (MW = 232 g/mole) may be undertaken with the following reactions: Fe3O4 → Fe2O3 → Fe (OH)3. Weight of sample containing 8.00% Fe3O4 that must be taken to obtain a precipitate of Fe(OH)3 (MW = 107 g/mole) that weighs 150 mg is . a. 0.108 g b. 0.325 g c. 1.355 g d. 4.065 g Amount of Fe2O3 (MW = 160 g/mole) from which 150 mg of Fe(OH)3 (MW = 107 g/mole) may be obtained is . a. 0.112 g b. 0.224 g c. 0.448 g d. none of the other choicesPlease show steps and states (aq),(s).. etc thank you5.00 mL of stock solution is diluted to 25.00 mL, producing solution ALPHA. 10.00 mL of solution ALPHA is diluted to 25.00 mL, resulting in solution BETA. 10.00 mL of solution BETA is then diluted to 25.00 mL, producing solution GAMMA. dilution factor for ALPHA from stock solution = 0.167 dilution factor for BETA from ALPHA solution = 0.0476 part c and d?
- Which of the following are equivalent to 2,500 ppm Cu2+? (There may be more than one answer) MW: Cu (63.55) a) 2.5 ppb Cu2+ b) 2,500,000 ppb Cu c) 2.5 ppt Cu2+ d) 39.34 mM e) 0.03934 M f) 0.07868 N (in precipitation reaction) g) 0.07868 N (in redox into Cu+)Aleks data for AgBrO3 is 5.38 x 10^-5Airbag experiment The simulation link is provided below if needed. It's probably best to use the simulation because the pictures attached won't show everything in full. Simulation link: https://interactives.ck12.org/simulations/chemistry/decomposition-reaction/app/index.html?screen=sandbox&lang=en&referrer=ck12Launcher&backUrl=https://interactives.ck12.org/simulations/chemistry.html Just select ammonium nitrate and then select 1 mole and press play. Record macroscopic observations of the bag and the crash test dummy as well as microscopic observations of the particles within the steering mechanism and the inflated/inflating airbag.

