Problems 1. What is the normality of a solution of H2SO4 if it has a specific gravity of 1.84 and contains 95.0% by weight. How many millimoles are contained in each milliliter? 2. If 30.00 ml of KHC204 H2C204 2H20 are dissolved, diluted in 1000ml, and it is found that 40.00 ml are neutralized by 20.00 mL of a solution of KOH. What is the normality of the alkali solution? 3. A mixture of 46.32 g of pure KOH and 27.64 g of NaOH is dissolved in water and diluted to 1000 ml. What volume of 1.022 N HCI is required to neutralize 50.00 mL of the solution? What volume of 1.022 M H2504 is required to neutralize 50.00 ml of the solution? 4. A 500-ml volumetric flask contains 150 mL of 0.200N H2S04. By adding a more concentrated H2504, the solution is brought up to the mark. After mixing, it is found to be 0.150 M. What was the N of the acid added? 5. What is the equivalent weight of a certain organic acid if 44.00 mL of NaOH solution (1.000 mL = 1.100 mL HCI = 0.01001 g CaCO3) are required to neutralize 0.5192 g of the acid? 6. Caustic potash that has been exposed to air is found on analysis to contain 90.00% KOH, 2.38% K2CO3 and 7.62% H2O. What weight of residue will be obtained if 1.00 g of this sample is added to 46.00 ml of 1.00 N HCI and the resulting solution, after neutralization with 1.070N KOH is evaporated to dryness? 7. In the analysis of a 2.000 g sample of lime by titration with H2S04, what must be the normality of the acid so that the percentage of Ca may be found by dividing the net volume of acid required by 4? 8. A sample known to consist of NaOH orNaHCO3, or Na2CO3 or possible compatible mixtures of these, together with inert matter. With methyl orange, a 1.10 g sample requires 31.40 mL of HCI (of which 1.00 mL = 0.0140 g Cao). With phenolphthalein, the same weight of sample requires 13.30 ml of the acid. Calculate the percentage of inert matter in the sample.
Problems 1. What is the normality of a solution of H2SO4 if it has a specific gravity of 1.84 and contains 95.0% by weight. How many millimoles are contained in each milliliter? 2. If 30.00 ml of KHC204 H2C204 2H20 are dissolved, diluted in 1000ml, and it is found that 40.00 ml are neutralized by 20.00 mL of a solution of KOH. What is the normality of the alkali solution? 3. A mixture of 46.32 g of pure KOH and 27.64 g of NaOH is dissolved in water and diluted to 1000 ml. What volume of 1.022 N HCI is required to neutralize 50.00 mL of the solution? What volume of 1.022 M H2504 is required to neutralize 50.00 ml of the solution? 4. A 500-ml volumetric flask contains 150 mL of 0.200N H2S04. By adding a more concentrated H2504, the solution is brought up to the mark. After mixing, it is found to be 0.150 M. What was the N of the acid added? 5. What is the equivalent weight of a certain organic acid if 44.00 mL of NaOH solution (1.000 mL = 1.100 mL HCI = 0.01001 g CaCO3) are required to neutralize 0.5192 g of the acid? 6. Caustic potash that has been exposed to air is found on analysis to contain 90.00% KOH, 2.38% K2CO3 and 7.62% H2O. What weight of residue will be obtained if 1.00 g of this sample is added to 46.00 ml of 1.00 N HCI and the resulting solution, after neutralization with 1.070N KOH is evaporated to dryness? 7. In the analysis of a 2.000 g sample of lime by titration with H2S04, what must be the normality of the acid so that the percentage of Ca may be found by dividing the net volume of acid required by 4? 8. A sample known to consist of NaOH orNaHCO3, or Na2CO3 or possible compatible mixtures of these, together with inert matter. With methyl orange, a 1.10 g sample requires 31.40 mL of HCI (of which 1.00 mL = 0.0140 g Cao). With phenolphthalein, the same weight of sample requires 13.30 ml of the acid. Calculate the percentage of inert matter in the sample.
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 109AE: Patients undergoing an upper gastrointestinal tract laboratory test are typically given an X-ray...
Related questions
Question

Transcribed Image Text:Problems
1. What is the normality of a solution of H2S04 if it has a specific gravity of 1.84 and contains 95.0%
by weight. How many millimoles are contained in each milliliter?
2. If 30.00 ml of KHC204 H2C204•2H20 are dissolved, diluted in 1000ml, and it is found that 40.00
ml are neutralized by 20.00 mL of a solution of KOH. What is the normality of the alkali solution?
3. A mixture of 46.32 g of pure KOH and 27.64 g of NaOH is dissolved in water and diluted to 1000
mL. What volume of 1.022 N HCI is required to neutralize 50.00 mL of the solution? What volume
of 1.022 M H2504 is required to neutralize 50.00 mL of the solution?
4. A 500-ml volumetric flask contains 150 ml of 0.20ON H2S04. By adding a more concentrated
H2SO4, the solution is brought up to the mark. After mixing, it is found to be 0.150 M. What was
the N of the acid added?
5. What is the equivalent weight of a certain organic acid if 44.00 ml of NAOH solution (1.000 ml =
1.100 ml HCI = 0.01001 g CaCO3) are required to neutralize 0.5192 g of the acid?
6. Caustic potash that has been exposed to air is found on analysis to contain 90.00% KOH, 2.38%
K2CO3 and 7.62% H2O. What weight of residue will be obtained if 1.00 g of this sample is added
to 46.00 ml of 1.00 N HCl and the resulting solution, after neutralization with 1.070N KOH is
evaporated to dryness?
7. In the analysis of a 2.000 g sample of lime by titration with H2S04, what must be the normality of
the acid so that the percentage of Ca may be found by dividing the net volume of acid required
by 4?
8. A sample known to consist of NaOH orNaHCO3, or Na2C03 or possible compatible mixtures of
these, together with inert matter. With methyl orange, a 1.10 g sample requires 31.40 mL of HCI
(of which 1.00 ml = 0.0140 g Cao). With phenolphthalein, the same weight of sample requires
13.30 ml of the acid. Calculate the percentage of inert matter in the sample.
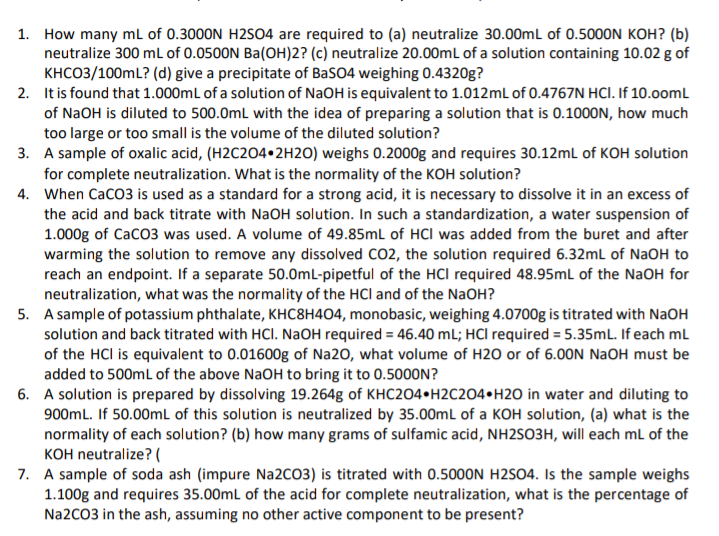
Transcribed Image Text:1. How many mL of 0.3000N H2S04 are required to (a) neutralize 30.00mL of 0.5000N KOH? (b)
neutralize 300 ml of 0.0500N Ba(OH)2? (c) neutralize 20.00mL of a solution containing 10.02 g of
KHCO3/100mL? (d) give a precipitate of BaSO4 weighing 0.4320g?
2. It is found that 1.000ml of a solution of NaOH is equivalent to 1.012mL of 0.4767N HCI. If 10.0omL
of NaOH is diluted to 500.0mL with the idea of preparing a solution that is 0.1000N, how much
too large or too small is the volume of the diluted solution?
3. A sample of oxalic acid, (H2C204•2H2O) weighs 0.2000g and requires 30.12ml of KOH solution
for complete neutralization. What is the normality of the KOH solution?
4. When CaCO3 is used as a standard for a strong acid, it is necessary to dissolve it in an excess of
the acid and back titrate with NaOH solution. In such a standardization, a water suspension of
1.000g of CaCO3 was used. A volume of 49.85mL of HCI was added from the buret and after
warming the solution to remove any dissolved CO2, the solution required 6.32ml of NaOH to
reach an endpoint. If a separate 50.0mL-pipetful of the HCl required 48.95mL of the NaOH for
neutralization, what was the normality of the HCl and of the NaOH?
5. A sample of potassium phthalate, KHC8H404, monobasic, weighing 4.0700g is titrated with NaOH
solution and back titrated with HCl. NaOH required = 46.40 mL; HCl required = 5.35mL. If each mL
of the HCl is equivalent to 0.01600g of Na20, what volume of H20 or of 6.00N NaOH must be
added to 500ml of the above NaOH to bring it to 0.5000N?
6. A solution is prepared by dissolving 19.264g of KHC2O4•H2C2O4•H2O in water and diluting to
900mL. If 50.00ml of this solution is neutralized by 35.00mL of a KOH solution, (a) what is the
normality of each solution? (b) how many grams of sulfamic acid, NH2SO3H, will each mL of the
KOH neutralize? (
7. A sample of soda ash (impure N22CO3) is titrated with 0.5000N H2S04. Is the sample weighs
1.100g and requires 35.00ml of the acid for complete neutralization, what is the percentage of
N22CO3 in the ash, assuming no other active component to be present?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning