Procedure Part 1: Obtain mass of a liquid in a beaker using weighing by difference technique. 1. Using the pictures in the table below, record the mass of the empty beaker and mass of the full beaker in the data table on the report sheet. Make sure to include units and the right number of significant figures. 2. Determine the mass of the liquid in the beaker for each trial. (m1+m2+m3+.mN) 3. Calculate the average mass in the beaker, using the expression mave trials performed. where N is the number of Trial Number Empty Beaker Full Beaker Trial 1 19.2 g 35.4 g On TARE Trial 2 20.0 g 36.1 g On TARE Trial 3 19.9 g 35.1 g
Procedure Part 1: Obtain mass of a liquid in a beaker using weighing by difference technique. 1. Using the pictures in the table below, record the mass of the empty beaker and mass of the full beaker in the data table on the report sheet. Make sure to include units and the right number of significant figures. 2. Determine the mass of the liquid in the beaker for each trial. (m1+m2+m3+.mN) 3. Calculate the average mass in the beaker, using the expression mave trials performed. where N is the number of Trial Number Empty Beaker Full Beaker Trial 1 19.2 g 35.4 g On TARE Trial 2 20.0 g 36.1 g On TARE Trial 3 19.9 g 35.1 g
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 2RCYU: A student checked the accuracy of two standard top-loading balances by testing them with a standard...
Related questions
Question
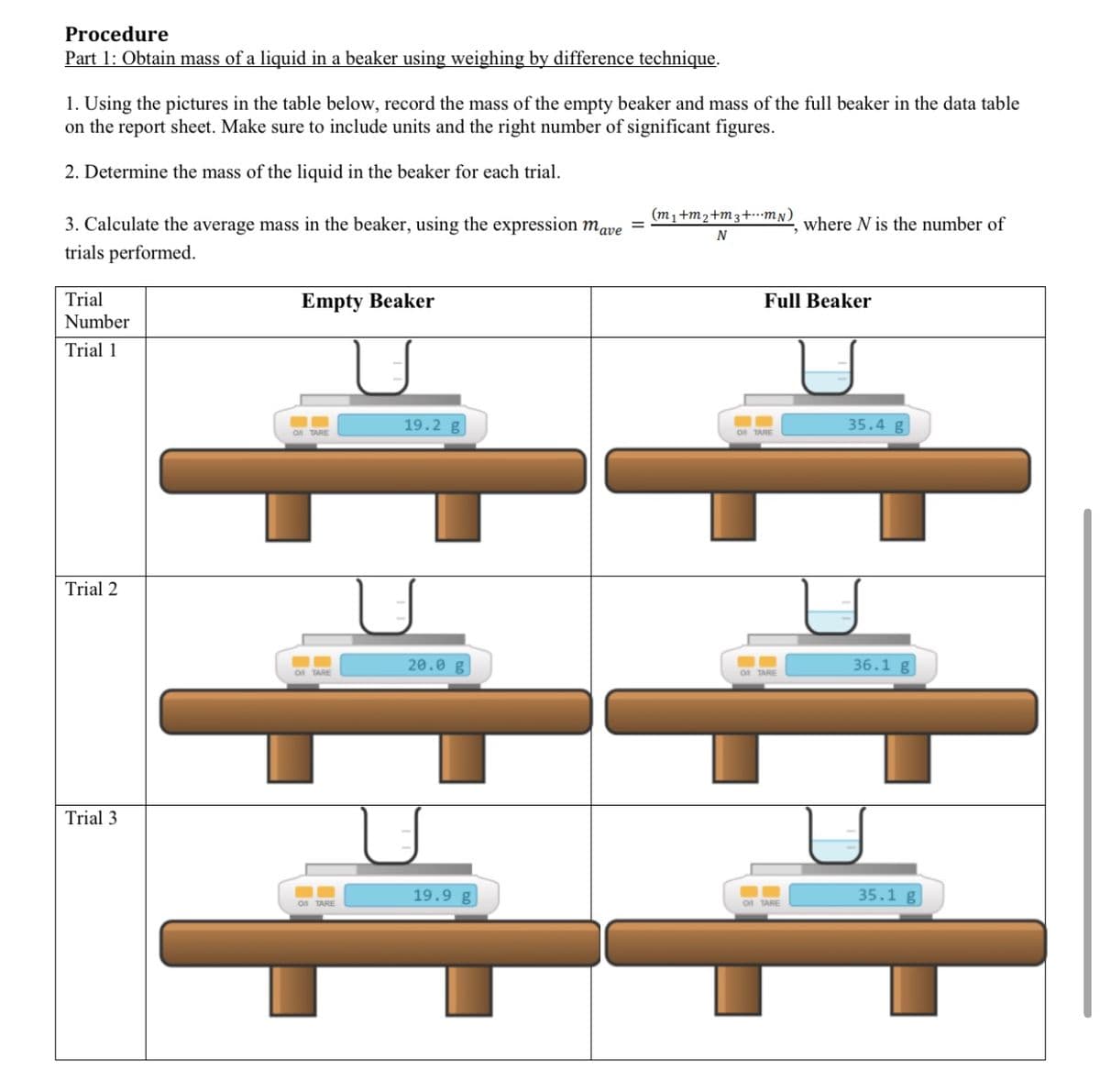
Transcribed Image Text:Procedure
Part 1: Obtain mass of a liquid in a beaker using weighing by difference technique.
1. Using the pictures in the table below, record the mass of the empty beaker and mass of the full beaker in the data table
on the report sheet. Make sure to include units and the right number of significant figures.
2. Determine the mass of the liquid in the beaker for each trial.
(m1+m2+m3+mN)
3. Calculate the average mass in the beaker, using the expression mave
trials performed.
where N is the number of
N
Trial
Empty Beaker
Full Beaker
Number
Trial 1
19.2 g
35.4 g
On TARE
On TARE
Trial 2
20.0 g
36.1 g
On TARE
On TARE
Trial 3
19.9 g
35.1 g
on TARE
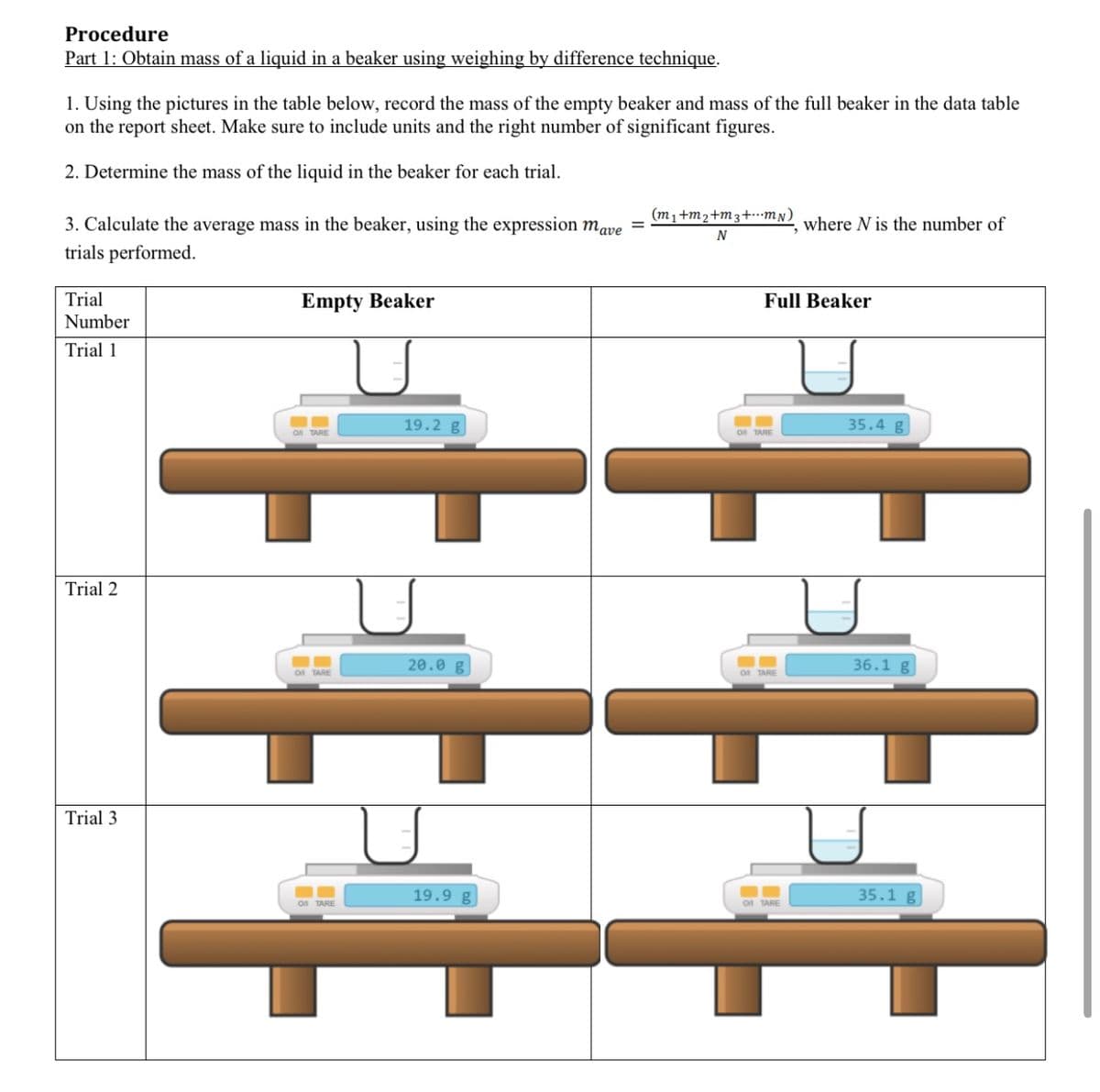
Transcribed Image Text:Procedure
Part 1: Obtain mass of a liquid in a beaker using weighing by difference technique.
1. Using the pictures in the table below, record the mass of the empty beaker and mass of the full beaker in the data table
on the report sheet. Make sure to include units and the right number of significant figures.
2. Determine the mass of the liquid in the beaker for each trial.
(m1+m2+m3+mN)
3. Calculate the average mass in the beaker, using the expression mave
trials performed.
where N is the number of
N
Trial
Empty Beaker
Full Beaker
Number
Trial 1
19.2 g
35.4 g
On TARE
On TARE
Trial 2
20.0 g
36.1 g
On TARE
On TARE
Trial 3
19.9 g
35.1 g
on TARE
Expert Solution
Step 1 Observation of the procedure
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning