Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 2.58 g of magnesium ibbon burns with 8.70 g of oxygen, a bright, white light and a white, powdery product are formed. Enter the balanced chemical equation for this reaction. Be sure to include all physical states. equation: 2Mg(s) + O2(g) - 2MGO(s) What is the limiting reactant? magnesium oxygen f the percent yield for the reaction is 83.6%, how many grams of product were formed? mass of product formed: 3.5767 How many grams of the excess reactant remain? 0.9516 mass of excess reactant: Incorrect
Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 2.58 g of magnesium ibbon burns with 8.70 g of oxygen, a bright, white light and a white, powdery product are formed. Enter the balanced chemical equation for this reaction. Be sure to include all physical states. equation: 2Mg(s) + O2(g) - 2MGO(s) What is the limiting reactant? magnesium oxygen f the percent yield for the reaction is 83.6%, how many grams of product were formed? mass of product formed: 3.5767 How many grams of the excess reactant remain? 0.9516 mass of excess reactant: Incorrect
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.19QP: You react nitrogen and hydrogen in a container to produce ammonia, NH3(g). The following figure...
Related questions
Question
What is the mass of the excess reactant?
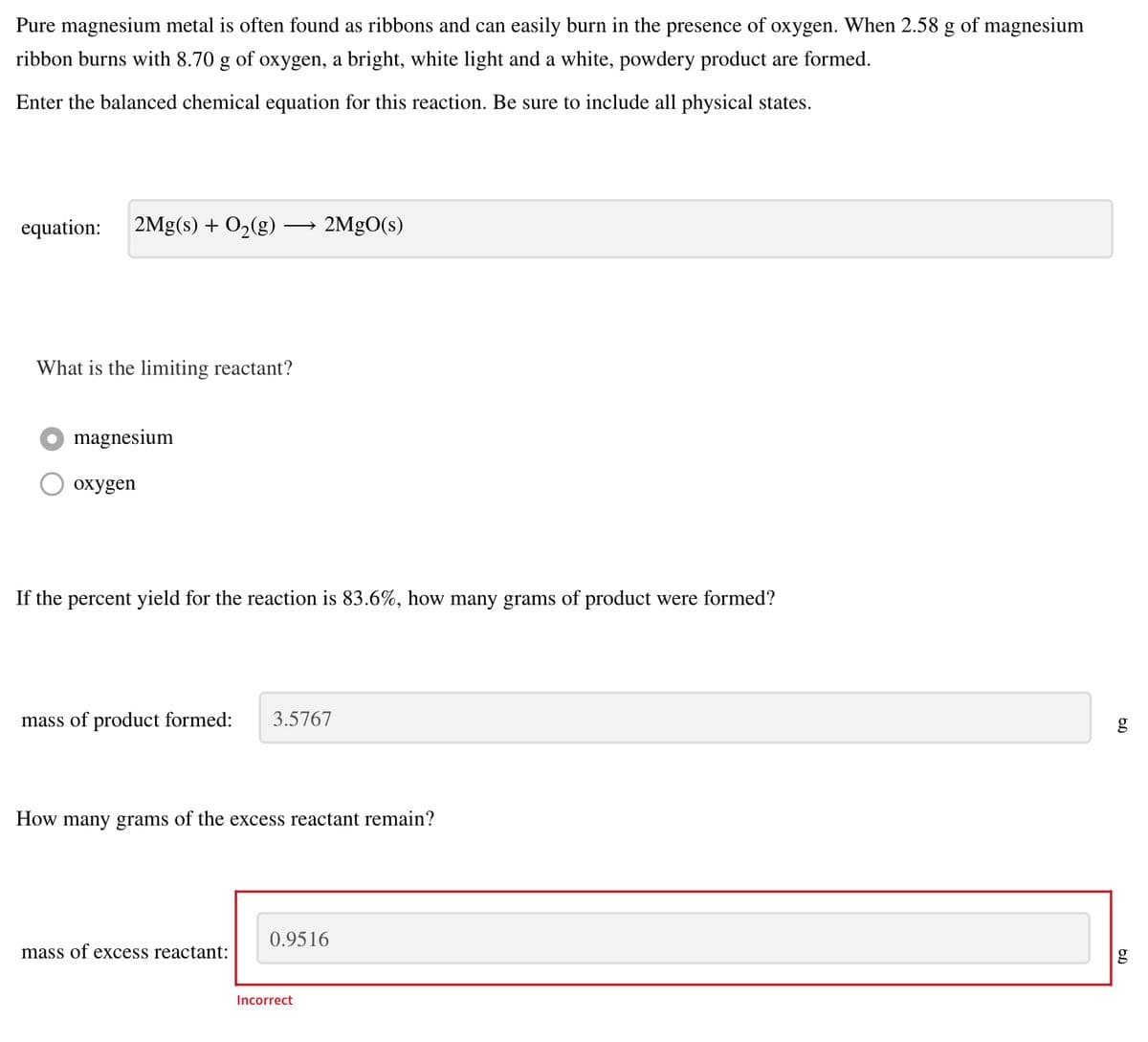
Transcribed Image Text:Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 2.58 g of magnesium
ribbon burns with 8.70
of
oxygen, a bright, white light and a white, powdery product are formed.
Enter the balanced chemical equation for this reaction. Be sure to include all physical states.
equation:
2Mg(s) + O2(g)·
2MgO(s)
What is the limiting reactant?
O magnesium
охудen
If the percent yield for the reaction is 83.6%, how many grams of product were formed?
mass of product formed:
3.5767
How many grams of the excess reactant remain?
0.9516
mass of excess reactant:
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning