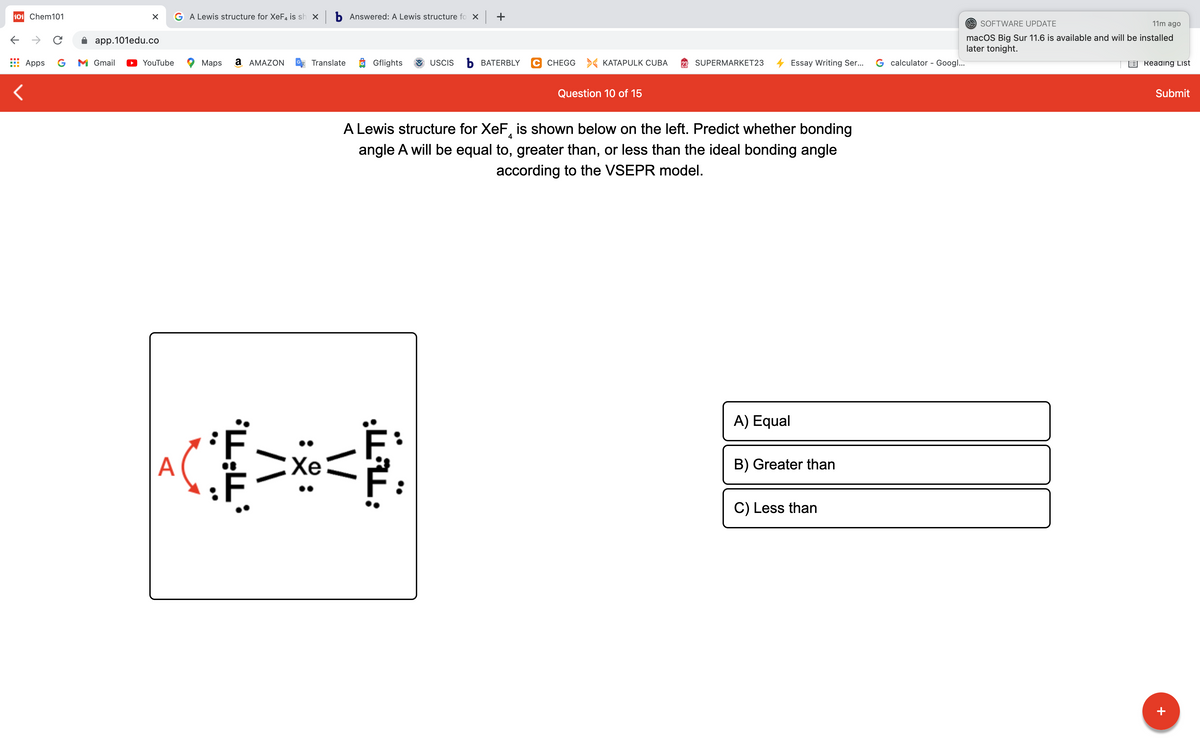
Question 10 of 15 A Lewis structure for XeF, is shown below on the left. Predict whether bonding angle A will be equal to, greater than, or less than the ideal bonding angle according to the VSEPR model. A) Equal B) Greater than C) Less than
Question 10 of 15 A Lewis structure for XeF, is shown below on the left. Predict whether bonding angle A will be equal to, greater than, or less than the ideal bonding angle according to the VSEPR model. A) Equal B) Greater than C) Less than
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.43QE: For each of the following molecules, complete the Lewis structure and use the VSEPR model to...
Related questions
Question
Transcribed Image Text:101 Chem101
G A Lewis structure for XeF4 is sh x b Answered: A Lewis structure fo X +
O SOFTWARE UPDATE
11m ago
macOS Big Sur 11.6 is available and will be installed
later tonight.
->
app.101edu.co
Apps
G
M Gmail
YouTube
Maps
a AMAZON
Translate
Gflights
USCIS
Ь ВАТERBLY
C CHEGG > KATAPULK CUBA
SUPERMARKET23
Essay Writing Ser...
G calculator - Googl...
E Reading List
Question 10 of 15
Submit
A Lewis structure for XeF, is shown below on the left. Predict whether bonding
4
angle A will be equal to, greater than, or less than the ideal bonding angle
according to the VSEPR model.
A) Equal
A
EXe
B) Greater than
C) Less than
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
