Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter1: Essential Ideas
Section: Chapter Questions
Problem 23E: When elemental iron corrodes it combines with oxygen in the air to ultimately form red brown...
Related questions
Question
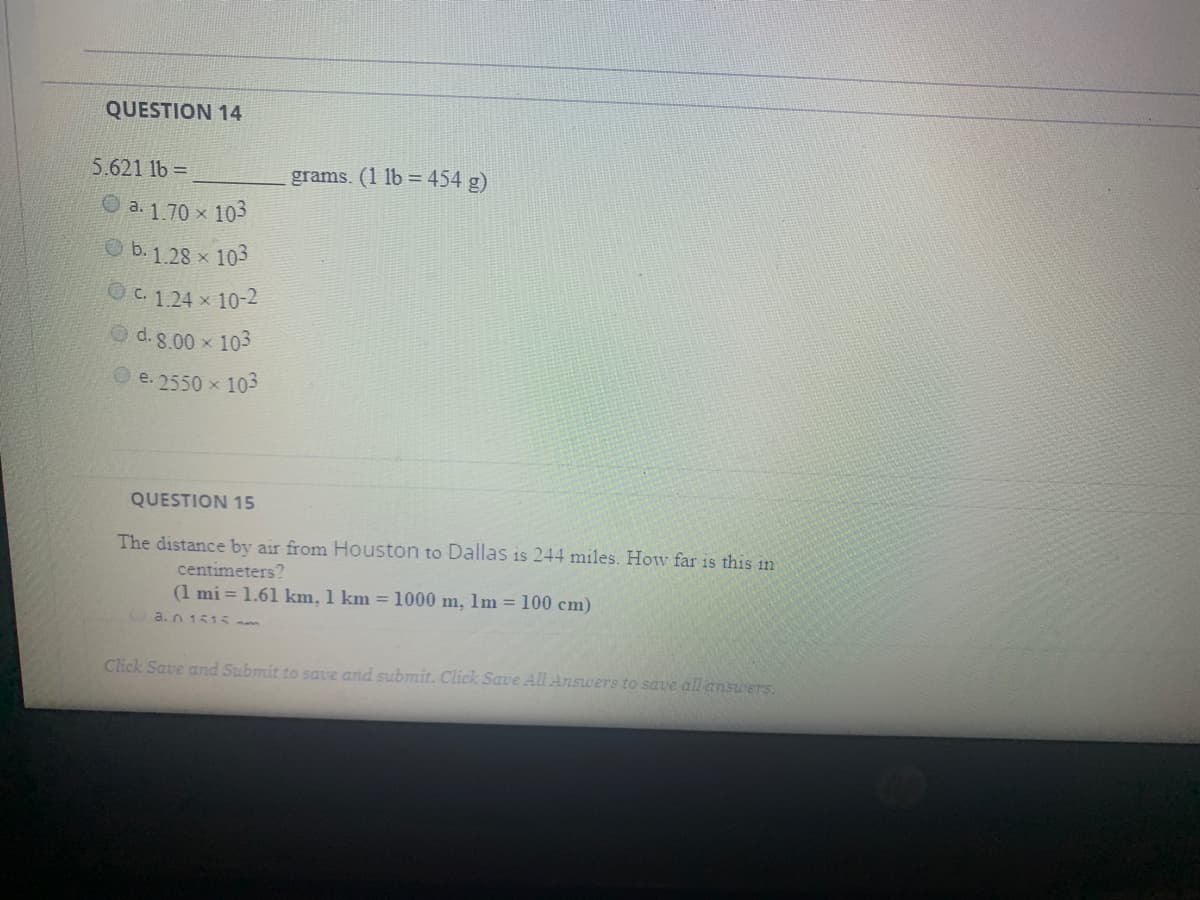
Transcribed Image Text:QUESTION 14
5.621 1b =
grams. (1 lb = 454 g)
O a. 1.70 x 103
b. 1.28 x 103
OC. 124 x 10-2
O d. 8.00 x 103
e. 2550 x 103
QUESTION 15
The distance by air from Houston to Dallas is 244 miles. How far is this in
centimeters?
(1 mi = 1.61 km, 1 km =1000 m, 1m = 100 cm)
a.n 1515 m
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Expert Solution
Step 1
Mass of any compound can be changed to different unit .
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning