Constants I Pe You may want to reference (Pages 258-262) section 6.4 while completing this problem. Part A An unknown mass of each of the following substances, initially at 25.0 O absorbs 1960 Jof heat. The final temperature is recordedas indicated. Find the mass of each substance a Pyrex glass (T: = 55.6 C) Express your answer using two significant figures. m3D Submit Previous Answers Request Answer X Incorrect; Try Again; 14 attempts remaining Part B sand (T = 62.3 °C). 国 acer
Constants I Pe You may want to reference (Pages 258-262) section 6.4 while completing this problem. Part A An unknown mass of each of the following substances, initially at 25.0 O absorbs 1960 Jof heat. The final temperature is recordedas indicated. Find the mass of each substance a Pyrex glass (T: = 55.6 C) Express your answer using two significant figures. m3D Submit Previous Answers Request Answer X Incorrect; Try Again; 14 attempts remaining Part B sand (T = 62.3 °C). 国 acer
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
Section: Chapter Questions
Problem 4STP
Related questions
Question
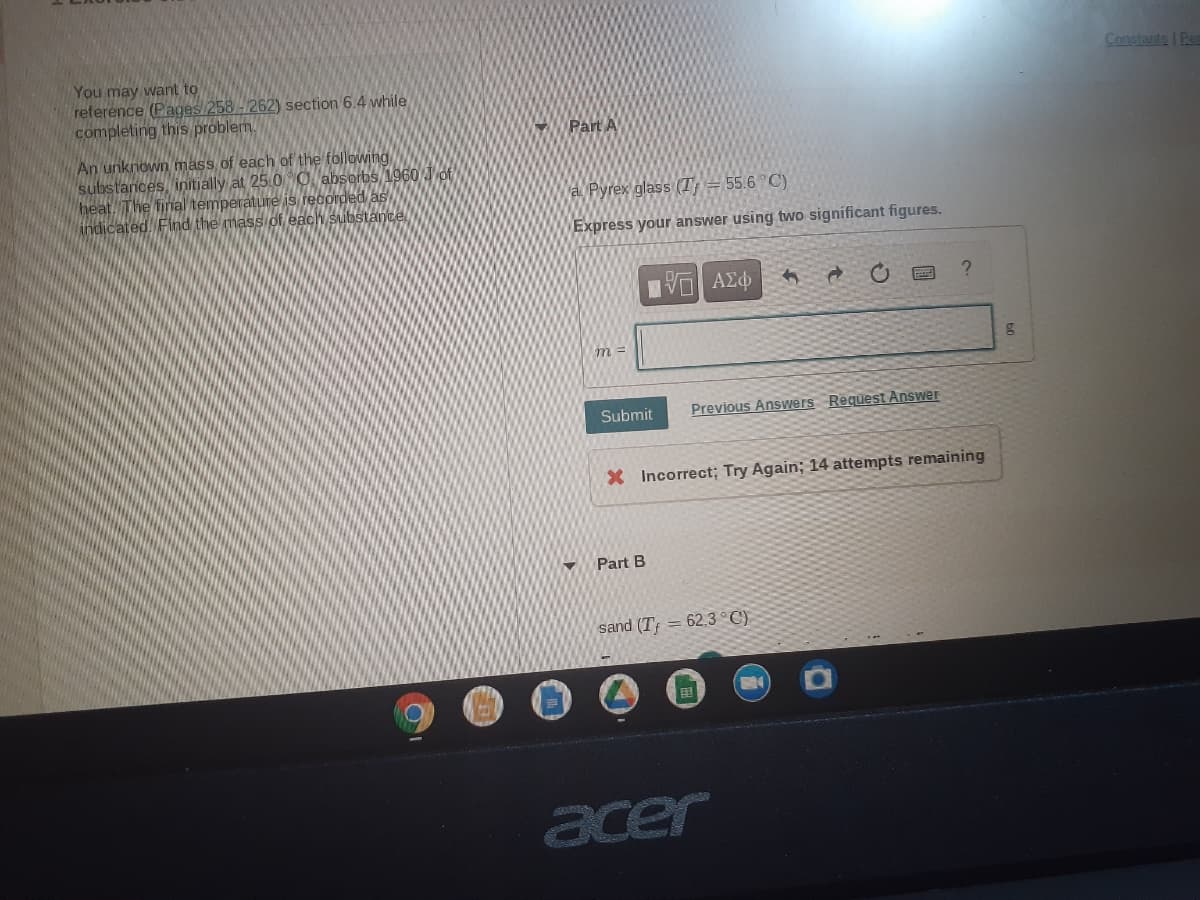
Transcribed Image Text:Constants | Ren
You may want to
reference (Pages 258 -262) section 6.4 while
completing this problem.
Part A
An unknown mass of each of the following
substances, initjally at 25.0 O absorbs 1960 Jof
heat. The fial temperature is recordedas
indicated. Find the mass of each substance
a Pyrex glass (T = 55.6°C)
Express your answer using two significant figures.
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 14 attempts remaining
Part B
sand (T; = 62.3 °C)
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,