Question 26 of 29 A sample of 14.2 g of Fe, O3 reacts with 13.9 g CO to yield Fe and CO,. The balanced chemical equation is 2 Fe,O3 (s) +3 CO(g)2 Fe(s) +3 CO, (g) Which substance is the limiting reactant? O Fe,O3 СО OFe O CO2 What is the theoretical yield of Fe? mass of Fe: If the actual experimental yield for Fe is 9.42 g, what is the percent yield of Fe? %e percent yield: help contact us terms of use privacy policy about us careers 60
Question 26 of 29 A sample of 14.2 g of Fe, O3 reacts with 13.9 g CO to yield Fe and CO,. The balanced chemical equation is 2 Fe,O3 (s) +3 CO(g)2 Fe(s) +3 CO, (g) Which substance is the limiting reactant? O Fe,O3 СО OFe O CO2 What is the theoretical yield of Fe? mass of Fe: If the actual experimental yield for Fe is 9.42 g, what is the percent yield of Fe? %e percent yield: help contact us terms of use privacy policy about us careers 60
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 60QAP: The text explains that one reason why the actual yield for a reaction may be less than the...
Related questions
Question
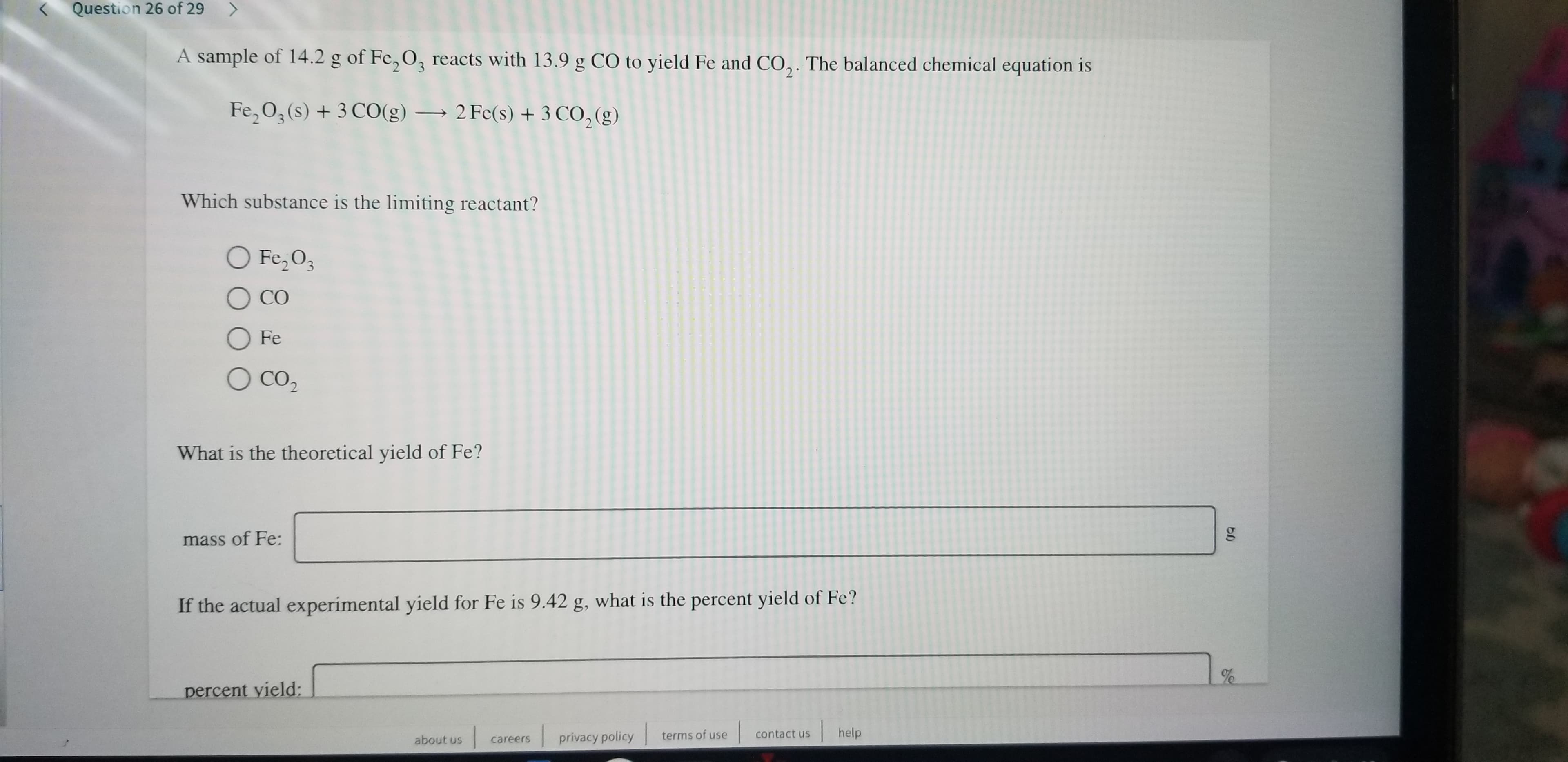
Transcribed Image Text:Question 26 of 29
A sample of 14.2 g of Fe, O3 reacts with 13.9 g CO to yield Fe and CO,. The balanced chemical equation is
2
Fe,O3 (s) +3 CO(g)2 Fe(s) +3 CO, (g)
Which substance is the limiting reactant?
O Fe,O3
СО
OFe
O CO2
What is the theoretical yield of Fe?
mass of Fe:
If the actual experimental yield for Fe is 9.42 g, what is the percent yield of Fe?
%e
percent yield:
help
contact us
terms of use
privacy policy
about us
careers
60
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 5 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning