Question 8 In the first asymmetric synthesis of (-)-(S,S)-homaline, a symmetric alkaloid isolated in the early 1970s, a key intermediate was compound 2 (Tetradhedron Lett. 2012, 53, 1119-1121). Provide the reagents for converting compound 1 into compound 2. Make the tertiary amine at first. `Ng Me. HN Two steps CO_Me .CO.Me Ph Ph Choose from the list of reagents: NH2 NH2 a. H3O°, NABH3CN b. H3O*, NABH3CN H3O", NABH3CN d. H3C H3O°, NABH;CN CH3 H30*, NABH,CN CH, f. H3O*, NABH3CN е. g. NazCr,07, H2SO4, H20 h. xs Crog, H3O", acetone i. PCC, CH2CI2 j. NABH4, MEOH k. 1) xs LAH 2) H20 I. NaN3 Step 1 f Step 2 I 22.90. Two steps are required. The secondary amine must be methylated to give a tertiary amine, and the halogen (CI) must be replaced with azide. The first step can be achieved via a reductive amination (the nitrogen atom cannot simply be methylated by using Mel, because that would result in over-alkylation, giving the quaternary salt, RaN* N. Then, in the second step, Cl can function as a leaving group in an Sy2 reaction with sodium azide, to afford compound 2. Me NaNg Me HN [H1 NABH,CN Co,Me .cOMe Ph Ph Ph 2
Question 8 In the first asymmetric synthesis of (-)-(S,S)-homaline, a symmetric alkaloid isolated in the early 1970s, a key intermediate was compound 2 (Tetradhedron Lett. 2012, 53, 1119-1121). Provide the reagents for converting compound 1 into compound 2. Make the tertiary amine at first. `Ng Me. HN Two steps CO_Me .CO.Me Ph Ph Choose from the list of reagents: NH2 NH2 a. H3O°, NABH3CN b. H3O*, NABH3CN H3O", NABH3CN d. H3C H3O°, NABH;CN CH3 H30*, NABH,CN CH, f. H3O*, NABH3CN е. g. NazCr,07, H2SO4, H20 h. xs Crog, H3O", acetone i. PCC, CH2CI2 j. NABH4, MEOH k. 1) xs LAH 2) H20 I. NaN3 Step 1 f Step 2 I 22.90. Two steps are required. The secondary amine must be methylated to give a tertiary amine, and the halogen (CI) must be replaced with azide. The first step can be achieved via a reductive amination (the nitrogen atom cannot simply be methylated by using Mel, because that would result in over-alkylation, giving the quaternary salt, RaN* N. Then, in the second step, Cl can function as a leaving group in an Sy2 reaction with sodium azide, to afford compound 2. Me NaNg Me HN [H1 NABH,CN Co,Me .cOMe Ph Ph Ph 2
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter16: Aldehydes And Ketones
Section: Chapter Questions
Problem 16.39P
Related questions
Question
Complete reaction mechanism (arrow pushing mechanism, resonance stabilization, charges)
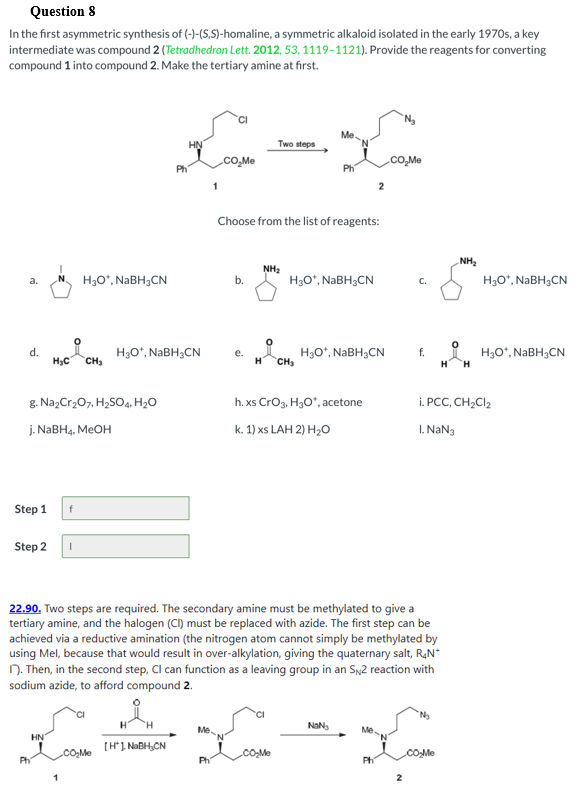
Transcribed Image Text:Question 8
In the first asymmetric synthesis of (-)-(S,S)-homaline, a symmetric alkaloid isolated in the early 1970s, a key
intermediate was compound 2 (Tetradhedron Lett. 2012, 53, 1119-1121). Provide the reagents for converting
compound 1 into compound 2. Make the tertiary amine at first.
Me
HN
Two steps
cO,Me
cO,Me
Ph
1
Choose from the list of reagents:
NH2
NH2
H3O*, NABH3CN
H3O*, NABH3CN
H3O°, NABH3CN
b.
C.
a.
d.
H3C
H3O*, NABH;CN
H3O*, NABH3CN
CH3
H3O“, NaBH3CN
H.
f.
е.
H
CH
H
g. NazCr207, H,SO4, H2O
h. xs Crog, H3O", acetone
i. PCC, CH2CI2
j. NABH4, MEOH
k. 1) xs LAH 2) H20
I. NaN3
Step 1
Step 2
22.90. Two steps are required. The secondary amine must be methylated to give a
tertiary amine, and the halogen (CI) must be replaced with azide. The first step can be
achieved via a reductive amination (the nitrogen atom cannot simply be methylated by
using Mel, because that would result in over-alkylation, giving the quaternary salt, RạN*
N. Then, in the second step, Cl can function as a leaving group in an Sy2 reaction with
sodium azide, to afford compound 2.
Me.
Me.
HN
[H1 NABH,CN
.co,Me
.cO.Me
cOMe
Ph
Ph
Ph
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
