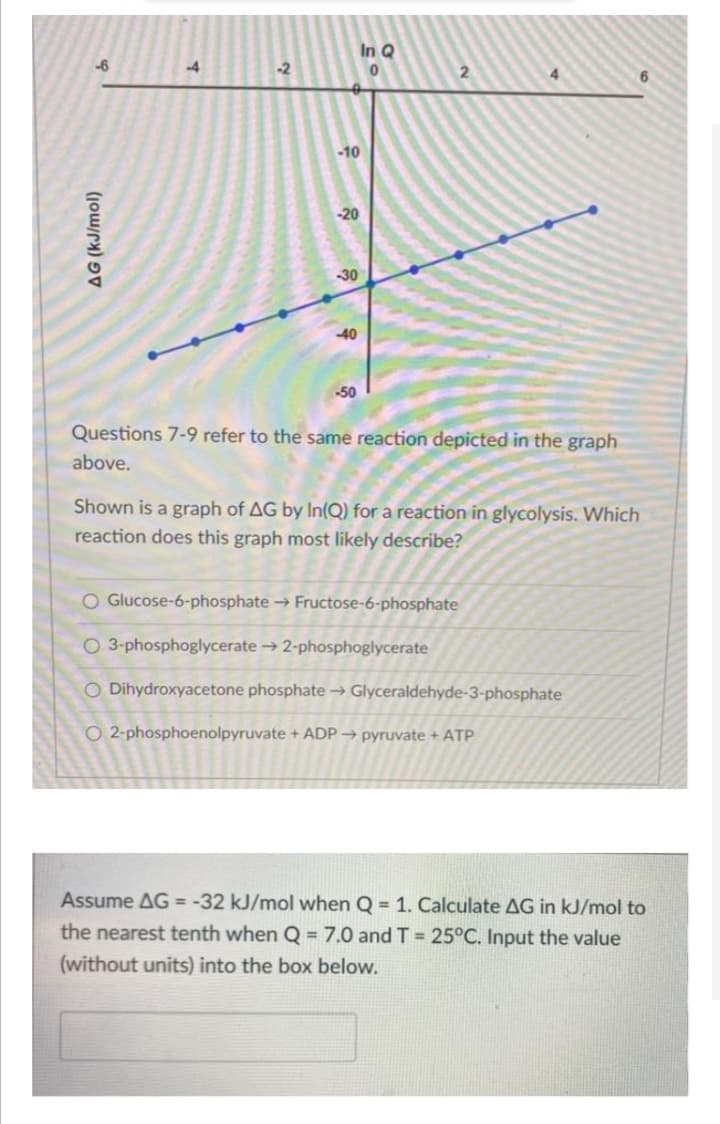
Questions 7-9 refer to the same reaction depicted in the graph above. Shown is a graph of AG by In(Q) for a reaction in glycolysis. Which reaction does this graph most likely describe? O Glucose-6-phosphate Fructose-6-phosphate O 3-phosphoglycerate → 2-phosphoglycerate O Dihydroxyacetone phosphate → Glyceraldehyde-3-phosphate O 2-phosphoenolpyruvate + ADP → pyruvate + ATP
Questions 7-9 refer to the same reaction depicted in the graph above. Shown is a graph of AG by In(Q) for a reaction in glycolysis. Which reaction does this graph most likely describe? O Glucose-6-phosphate Fructose-6-phosphate O 3-phosphoglycerate → 2-phosphoglycerate O Dihydroxyacetone phosphate → Glyceraldehyde-3-phosphate O 2-phosphoenolpyruvate + ADP → pyruvate + ATP
Chapter29: The Organic Chemistry Of Metabolic Pathways
Section29.SE: Something Extra
Problem 22MP
Related questions
Question
Transcribed Image Text:In Q
-6
4
-2
-10
-20
-30
40
-50
Questions 7-9 refer to the same reaction depicted in the graph
ove.
Shown is a graph of AG by In(Q) for a reaction in glycolysis. Which
reaction does this graph most likely describe?
O Glucose-6-phosphate → Fructose-6-phosphate
O 3-phosphoglycerate → 2-phosphoglycerate
O Dihydroxyacetone phosphate → Glyceraldehyde-3-phosphate
O 2-phosphoenolpyruvate + ADP → pyruvate + ATP
Assume AG = -32 kJ/mol when Q = 1. Calculate AG in kJ/mol to
the nearest tenth when Q = 7.0 and T =25°C. Input the value
(without units) into the box below.
AG (kJ/mol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning