Referring to the figure below, we see that the atomic number Z is proportional to f/2 or that z² is proportional to f. Because the frequency of the characteristic x-ray lines is itself proportional to the energy of the associated x-ray photon, we are led to conclude that AE and hence the energies of the atomic levels also scale as z?. Based on this analysis (and ignoring any differences from the masses of their nuclei), how much greater is the energy associated with the ground state of boron (Z = 5) than that of hydrogen (Z = 1)? E = Make a similar comparison between the ground state energies for magnesium (Z = 12) and hydrogen. EMg = EH 48 Ka 42 36 30 - 24 18 - 12 6 10 20 30 40 50 60 70 80 90 100 Atomic number (Z)
Referring to the figure below, we see that the atomic number Z is proportional to f/2 or that z² is proportional to f. Because the frequency of the characteristic x-ray lines is itself proportional to the energy of the associated x-ray photon, we are led to conclude that AE and hence the energies of the atomic levels also scale as z?. Based on this analysis (and ignoring any differences from the masses of their nuclei), how much greater is the energy associated with the ground state of boron (Z = 5) than that of hydrogen (Z = 1)? E = Make a similar comparison between the ground state energies for magnesium (Z = 12) and hydrogen. EMg = EH 48 Ka 42 36 30 - 24 18 - 12 6 10 20 30 40 50 60 70 80 90 100 Atomic number (Z)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 135QRT
Related questions
Question
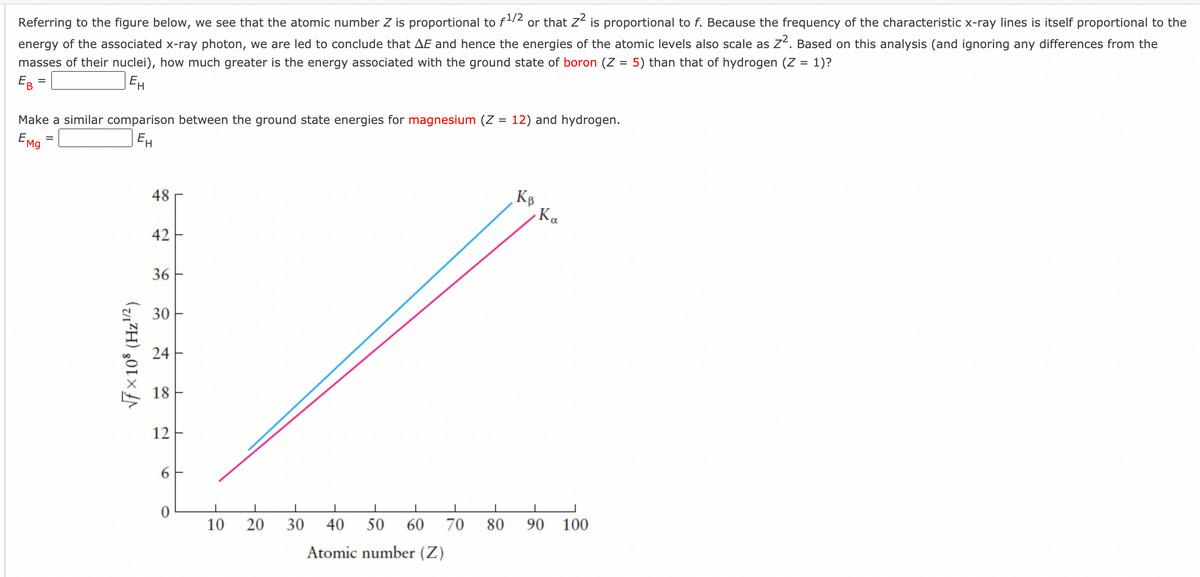
Transcribed Image Text:energy of the associated x-ray photon, we are led to conclude that AE and hence the energies of the atomic levels also scale as Z4. Based on this analysis (and ignoring any differences from the
masses of their nuclei), how much greater is the energy associated with the ground state of boron (Z = 5) than that of hydrogen (Z = 1)?
EB
Referring to the figure below, we see that the atomic number Z is proportional to f12 or that z“ is proportional to f. Because the frequency of the characteristic x-ray lines is itself proportional to the
EH
Make a similar comparison between the ground state energies for magnesium (Z = 12) and hydrogen.
EMg
H.
KB
Ka
48
42
36
30
24
18
12
6
10
30
40
50
60
70
80
90
100
Atomic number (Z)
20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning