Resources Lx Give Up? E Feedback Resum ion 22 of 30 > Attempt : Classify each substance based on the intermolecular forces present in that substance. Hydrogen bonding, dipole-dipole, and dispersion Dipole-dipole and dispersion only Dispersion only H,0 CH,CI CO, CO Answer Bank Incorrect
Resources Lx Give Up? E Feedback Resum ion 22 of 30 > Attempt : Classify each substance based on the intermolecular forces present in that substance. Hydrogen bonding, dipole-dipole, and dispersion Dipole-dipole and dispersion only Dispersion only H,0 CH,CI CO, CO Answer Bank Incorrect
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 87AP
Related questions
Question
Transcribed Image Text:O Resources
Ex Give Up?
E Feedback
nment Score:
42.8%
Resume
Lion 22 of 30 >
O Attempt 2
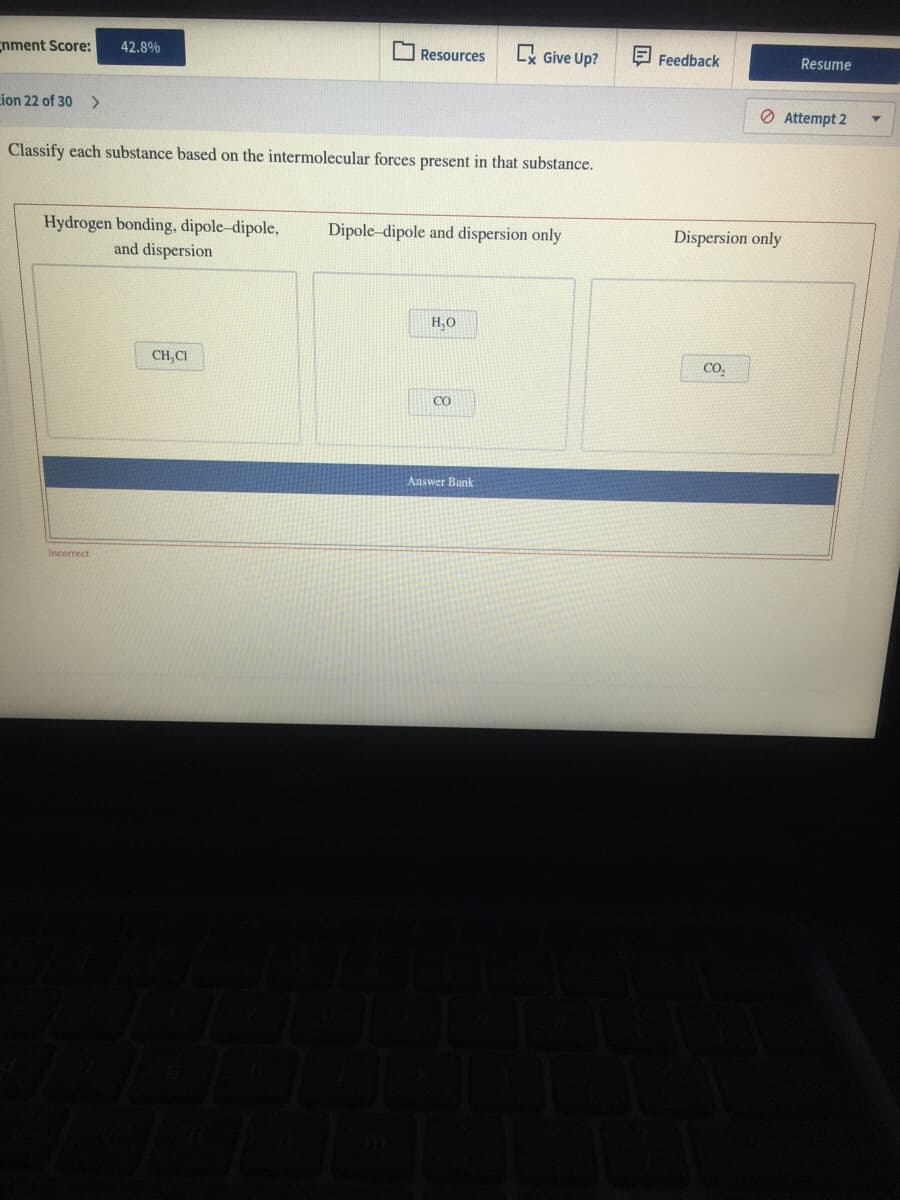
Classify each substance based on the intermolecular forces present in that substance.
Hydrogen bonding, dipole-dipole,
and dispersion
Dipole-dipole and dispersion only
Dispersion only
H,0
CH,CI
CO,
CO
Answer Bank
Incorrect
Expert Solution
Step 1
(1) Hydrogen bonding, dipole dipole and dispersion -
Hydrogen bonding present in water Molecule, it also has dipole due to difference in electronegativities of oxygen and hydrogen. Also in it's gaseous or Vapour form it has dispersion intermolecular forces. That why H2O has all these forces.
(2) Dipole dipole and dispersion only -
These two forces present in CH3Cl and CO because of difference in electronegativities between Carbon and Chlorine in chloromethane while between Carbon and oxygen in Carbon monoxide. They also contain dispersion or London forces.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax