Review Part B What is the pH after 0.150 mol of HCl is added to the buffer from Part A? Assume no volume change on the addition of the acid. Express the pH numerically to three decimal places. > View Available Hint(s) pH = You have already submitted this answer. Enter a new answer. No credit lost. Try again. Submit Previous Answers Request Answer Part C What is the pH after 0.195 mol of NaOH is added to the buffer from Part A? Assume no volume change on the addition of the base. Express the pH numerically to three decimal places. • View Available Hint(s) pH =
Review Part B What is the pH after 0.150 mol of HCl is added to the buffer from Part A? Assume no volume change on the addition of the acid. Express the pH numerically to three decimal places. > View Available Hint(s) pH = You have already submitted this answer. Enter a new answer. No credit lost. Try again. Submit Previous Answers Request Answer Part C What is the pH after 0.195 mol of NaOH is added to the buffer from Part A? Assume no volume change on the addition of the base. Express the pH numerically to three decimal places. • View Available Hint(s) pH =
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 51P
Related questions
Question
I need help for number 5 part b and c
![I Review | Constants | Periodic Table
When a solution contains a weak acid and its conjugate base or a
weak base and its conjugate acid, it will be a buffer solution.
Buffers resist change in pH following the addition of acid or base.
A butfer solution prepared from a weak acid (HA) and its.
conjugate base (A) is represented as
Part A
What is the pH of a buffer prepared by adding 0.809 mol of the weak acid HA to 0.305 mol of NaA in 2.00L of solution? The dissociation constant K. of
HA is 5.66 x 107
HA(aq) - H*(aq) + A (aq)
The buffer will follow Le Châtelier's principle. If acid is added, the
reaction shifts to consume the added H, forming more HA.
When base is added, the base will react with H, reducing its
concentration. The reaction then shifts to replace Ht through the
dissociation of HA into H and A":In both instances, H
Express the pH numerically to three decimal places.
> View Available Hint(s)
pH = 5.823
tends to remain constant.
Submit
The pH of a buffer is calculated by using the Henderson-
Hasselbalch equation:
Previous Answers
A]
v Correct
pH = pK, + log
HA
Since both the acid and base exist in the same volume, we can skip the concentration calculations and use the number of moles in the
Henderson-Hasselbalch equation to calculate the pH. The answer will be the same.
Part B
What is the pH after 0.150 mol of HCl is added to the buffer from Part A? Assume no volume change on the addition of the acid.
Express the pH numerically to three decimal places.
> View Available Hint(s)](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F01604a07-1abb-47f3-85be-4093016fdea3%2Fc0c8d7ee-1bfe-4082-9bf2-94bc74452272%2Fiytp0d9_processed.jpeg&w=3840&q=75)
Transcribed Image Text:I Review | Constants | Periodic Table
When a solution contains a weak acid and its conjugate base or a
weak base and its conjugate acid, it will be a buffer solution.
Buffers resist change in pH following the addition of acid or base.
A butfer solution prepared from a weak acid (HA) and its.
conjugate base (A) is represented as
Part A
What is the pH of a buffer prepared by adding 0.809 mol of the weak acid HA to 0.305 mol of NaA in 2.00L of solution? The dissociation constant K. of
HA is 5.66 x 107
HA(aq) - H*(aq) + A (aq)
The buffer will follow Le Châtelier's principle. If acid is added, the
reaction shifts to consume the added H, forming more HA.
When base is added, the base will react with H, reducing its
concentration. The reaction then shifts to replace Ht through the
dissociation of HA into H and A":In both instances, H
Express the pH numerically to three decimal places.
> View Available Hint(s)
pH = 5.823
tends to remain constant.
Submit
The pH of a buffer is calculated by using the Henderson-
Hasselbalch equation:
Previous Answers
A]
v Correct
pH = pK, + log
HA
Since both the acid and base exist in the same volume, we can skip the concentration calculations and use the number of moles in the
Henderson-Hasselbalch equation to calculate the pH. The answer will be the same.
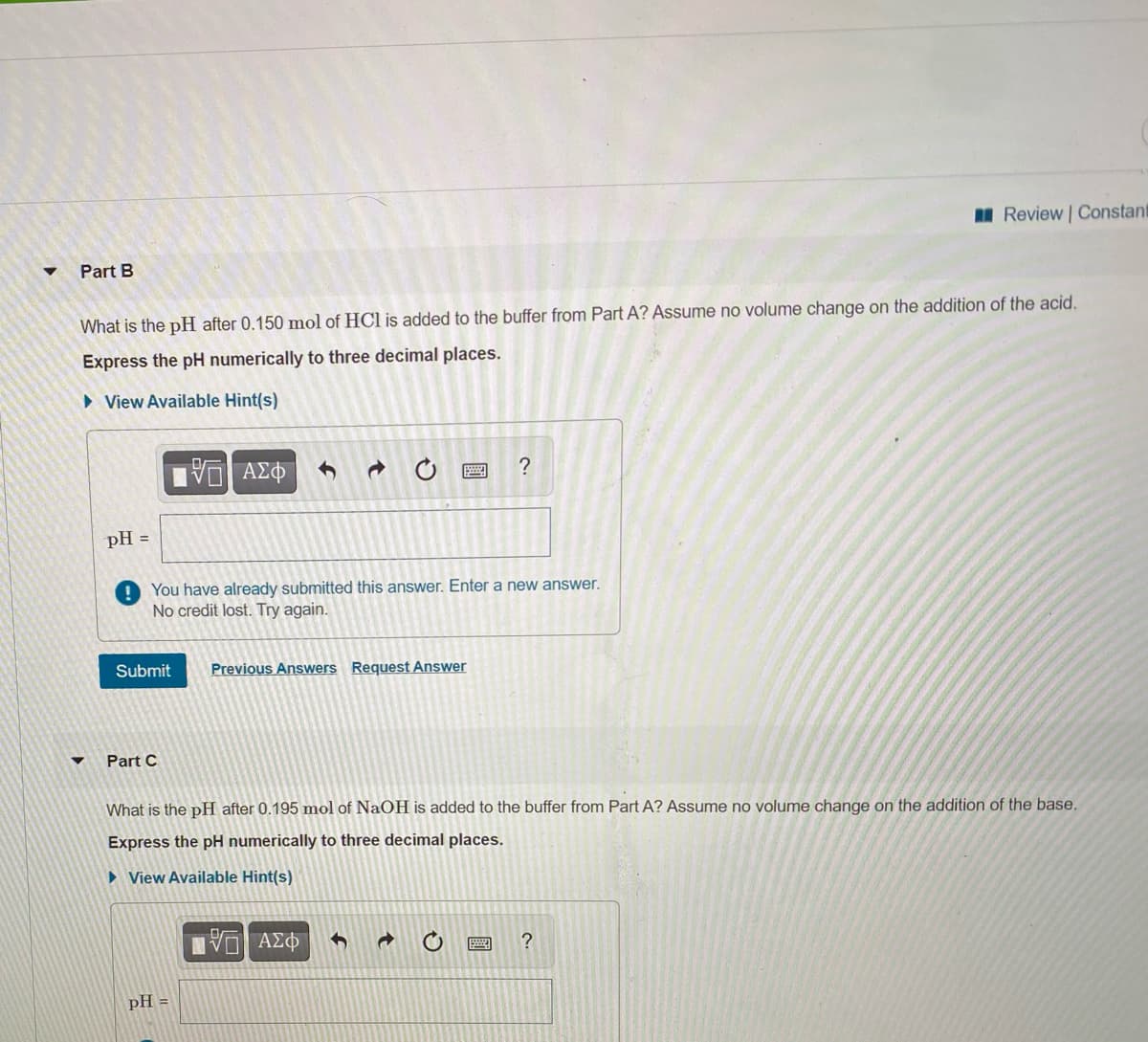
Part B
What is the pH after 0.150 mol of HCl is added to the buffer from Part A? Assume no volume change on the addition of the acid.
Express the pH numerically to three decimal places.
> View Available Hint(s)
Transcribed Image Text:I Review Constant
Part B
What is the pH after 0.150 mol of HCl is added to the buffer from Part A? Assume no volume change on the addition of the acid.
Express the pH numerically to three decimal places.
» View Available Hint(s)
ΑΣΦ
pH =
You have already submitted this answer. Enter a new answer.
No credit lost. Try again.
Submit
Previous Answers Request Answer
Part C
What is the pH after 0.195 mol of NaOH is added to the buffer from Part A? Assume no volume change on the addition of the base.
Express the pH numerically to three decimal places.
» View Available Hint(s)
VO AEO
?
pH =
國
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning