In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell of an atom is called the electron-pair geometry. A. The Lewis diagram for CS2 is: S=c=S Recall that for predicting geometry, double and triple bonds count as only one electron pair region. The electron-pair geometry around the C atom in CS, is There are unshared pair(s) around the central atom, so the geometry of the CS, molecule is B. The Lewis diagram for NO, is: 0-N=ö: Recall that for predicting geometry, double and triple bonds count as only one electron pair region. The electron-pair geometry around the N atom in NO2" is There are unshared pair(s) around the central atom, so the geometry of the NO," molecule is
In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell of an atom is called the electron-pair geometry. A. The Lewis diagram for CS2 is: S=c=S Recall that for predicting geometry, double and triple bonds count as only one electron pair region. The electron-pair geometry around the C atom in CS, is There are unshared pair(s) around the central atom, so the geometry of the CS, molecule is B. The Lewis diagram for NO, is: 0-N=ö: Recall that for predicting geometry, double and triple bonds count as only one electron pair region. The electron-pair geometry around the N atom in NO2" is There are unshared pair(s) around the central atom, so the geometry of the NO," molecule is
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 94AP: The molecular ion S3N3 has the cyclic structure All SN bonds are equivalent. (a) Give six...
Related questions
Question
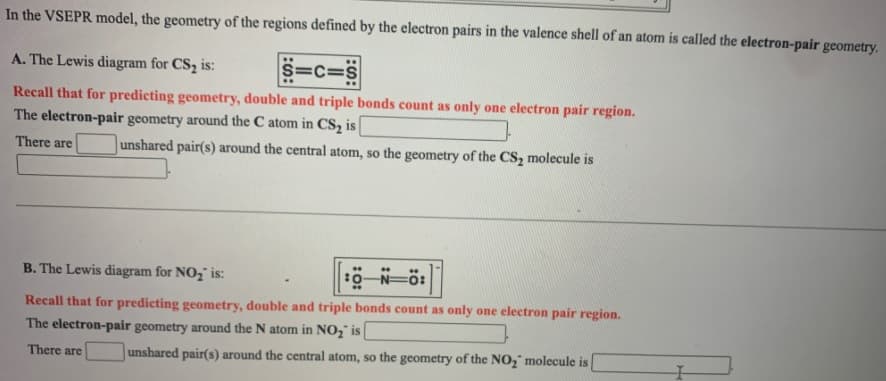
Transcribed Image Text:In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell of an atom is called the electron-pair geometry.
A. The Lewis diagram for CS2 is:
S=c=S
Recall that for predicting geometry, double and triple bonds count as only one electron pair region.
The electron-pair geometry around the C atom in CS, is
There are
unshared pair(s) around the central atom, so the geometry of the CS, molecule is
B. The Lewis diagram for NO, is:
0-N=ö:
Recall that for predicting geometry, double and triple bonds count as only one electron pair region.
The electron-pair geometry around the N atom in NO2" is
There are
unshared pair(s) around the central atom, so the geometry of the NO," molecule is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
