Show how these pairs of elements combine into octet-rule molecular compounds, as in the first example. First, draw their atomic Lewis structures, then use any number of these atoms to build an O.R. molecule, with multiple bonds if needed (you may not add a charge to make it O.R. as an ion!). Draw the O.R. Lewis structure, write its formula and chemical name. Atoms O.R. molecule Formula Name ö=c=ö Carbon dioxide + CO2 + F B + O
Show how these pairs of elements combine into octet-rule molecular compounds, as in the first example. First, draw their atomic Lewis structures, then use any number of these atoms to build an O.R. molecule, with multiple bonds if needed (you may not add a charge to make it O.R. as an ion!). Draw the O.R. Lewis structure, write its formula and chemical name. Atoms O.R. molecule Formula Name ö=c=ö Carbon dioxide + CO2 + F B + O
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter2: Lewis Structures
Section: Chapter Questions
Problem 6CTQ: It is impossible to draw a legitimate Lewis structure of a neutral NH4 molecule. Hypothetically,how...
Related questions
Question
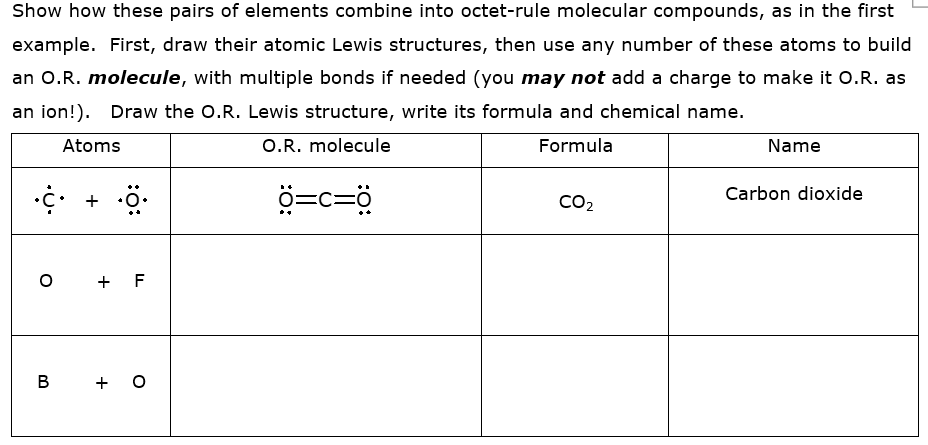
Transcribed Image Text:Show how these pairs of elements combine into octet-rule molecular compounds, as in the first
example. First, draw their atomic Lewis structures, then use any number of these atoms to build
an O.R. molecule, with multiple bonds if needed (you may not add a charge to make it O.R. as
an ion!). Draw the O.R. Lewis structure, write its formula and chemical name.
Atoms
O.R. molecule
Formula
Name
ö. + 5.
ö=c=ö
Carbon dioxide
CO2
o + F
B
+ O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

