MCB pectrophotometry Pre-lab Question: Calculations for Pro Standard Curve Please complete the table below and show ALL calculations in your notebook! Note that the stock solution of Lysozyme is 2.0mg/mL LYSOZYME STANDARDS Tube Volume Volume Final Final Amount of of (HL) Volume LysozymeLysozyme dH20 (HL) 2.0mg/mL | Concentration (ug/mL) (in μg) in 100 μし of (uL) Lysozyme stock 100 μしof | 100 μし | 2,000 μg/mL | 200 pg stock stock stock stock stock stock stock stock
MCB pectrophotometry Pre-lab Question: Calculations for Pro Standard Curve Please complete the table below and show ALL calculations in your notebook! Note that the stock solution of Lysozyme is 2.0mg/mL LYSOZYME STANDARDS Tube Volume Volume Final Final Amount of of (HL) Volume LysozymeLysozyme dH20 (HL) 2.0mg/mL | Concentration (ug/mL) (in μg) in 100 μし of (uL) Lysozyme stock 100 μしof | 100 μし | 2,000 μg/mL | 200 pg stock stock stock stock stock stock stock stock
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 1P
Related questions
Question
Can I get some help doing these? If I can see one example done I could do the rest
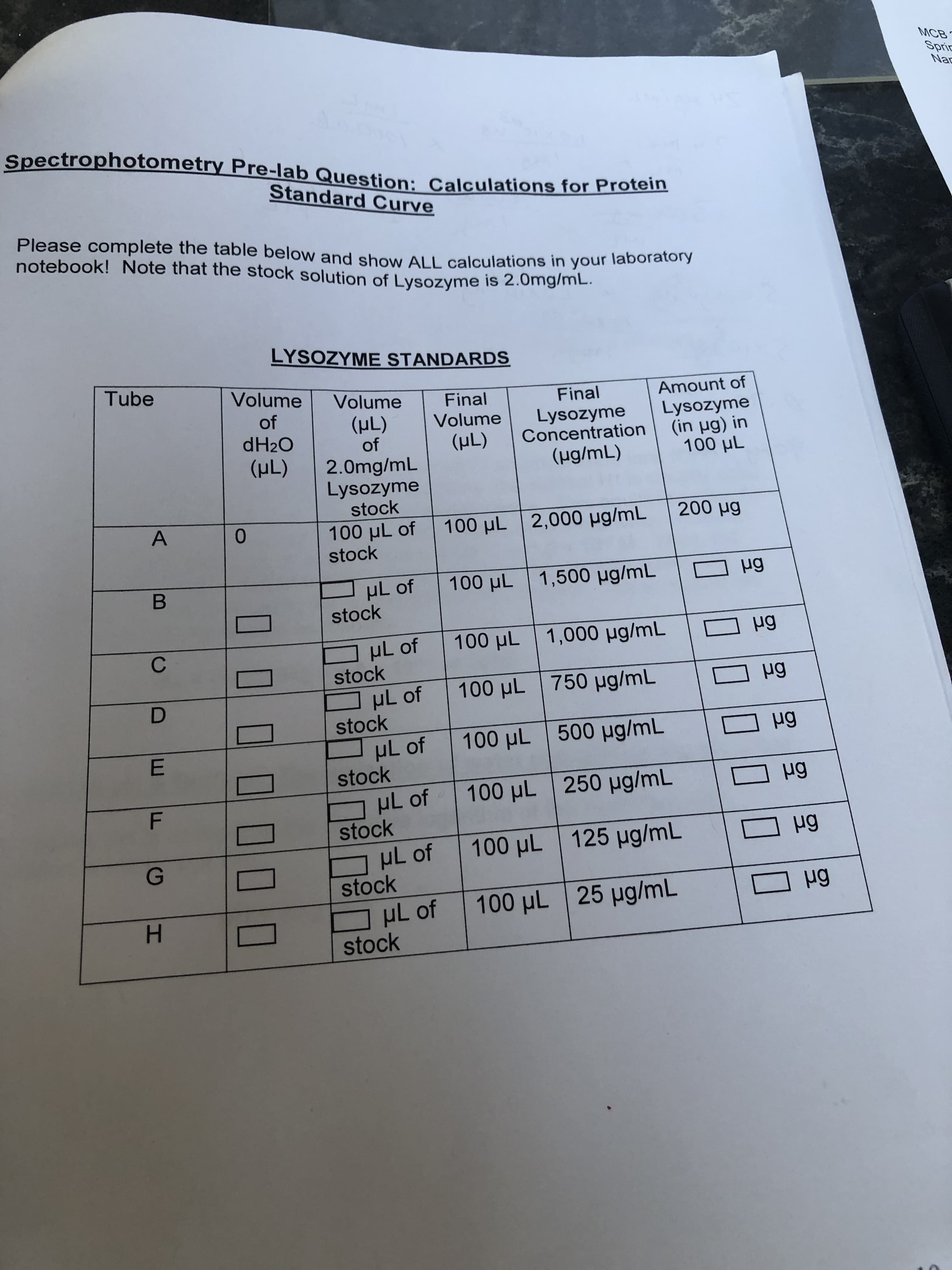
Transcribed Image Text:MCB
pectrophotometry Pre-lab Question: Calculations for Pro
Standard Curve
Please complete the table below and show ALL calculations in your
notebook! Note that the stock solution of Lysozyme is 2.0mg/mL
LYSOZYME STANDARDS
Tube
Volume Volume Final
Final
Amount of
of
(HL) Volume LysozymeLysozyme
dH20
(HL) 2.0mg/mL
| Concentration
(ug/mL)
(in μg) in
100 μし
of
(uL)
Lysozyme
stock
100 μしof | 100 μし | 2,000 μg/mL
| 200 pg
stock
stock
stock
stock
stock
stock
stock
stock
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT