Silicon carbide (SiC) is an extremely hard substance used in bullet proof vests and other hard materials. It has a very high melting point and is a poor conductor of electricity. What kinds of bonding do you think exist across the SiC structure? Covalent pi bonds Metallic-type electron sharing Covalent sigma bonds Covalent sigma and pi bonds London Dispersive Forces It is impossible to answer this question only considering the physical properties of the compound
Silicon carbide (SiC) is an extremely hard substance used in bullet proof vests and other hard materials. It has a very high melting point and is a poor conductor of electricity. What kinds of bonding do you think exist across the SiC structure? Covalent pi bonds Metallic-type electron sharing Covalent sigma bonds Covalent sigma and pi bonds London Dispersive Forces It is impossible to answer this question only considering the physical properties of the compound
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter7: Chemical Bonding And Molecular Structure
Section: Chapter Questions
Problem 7.13PAE: 7.13 Figure 7-2 depicts the interactions of an ion with its first nearest neighbors, second nearest...
Related questions
Question
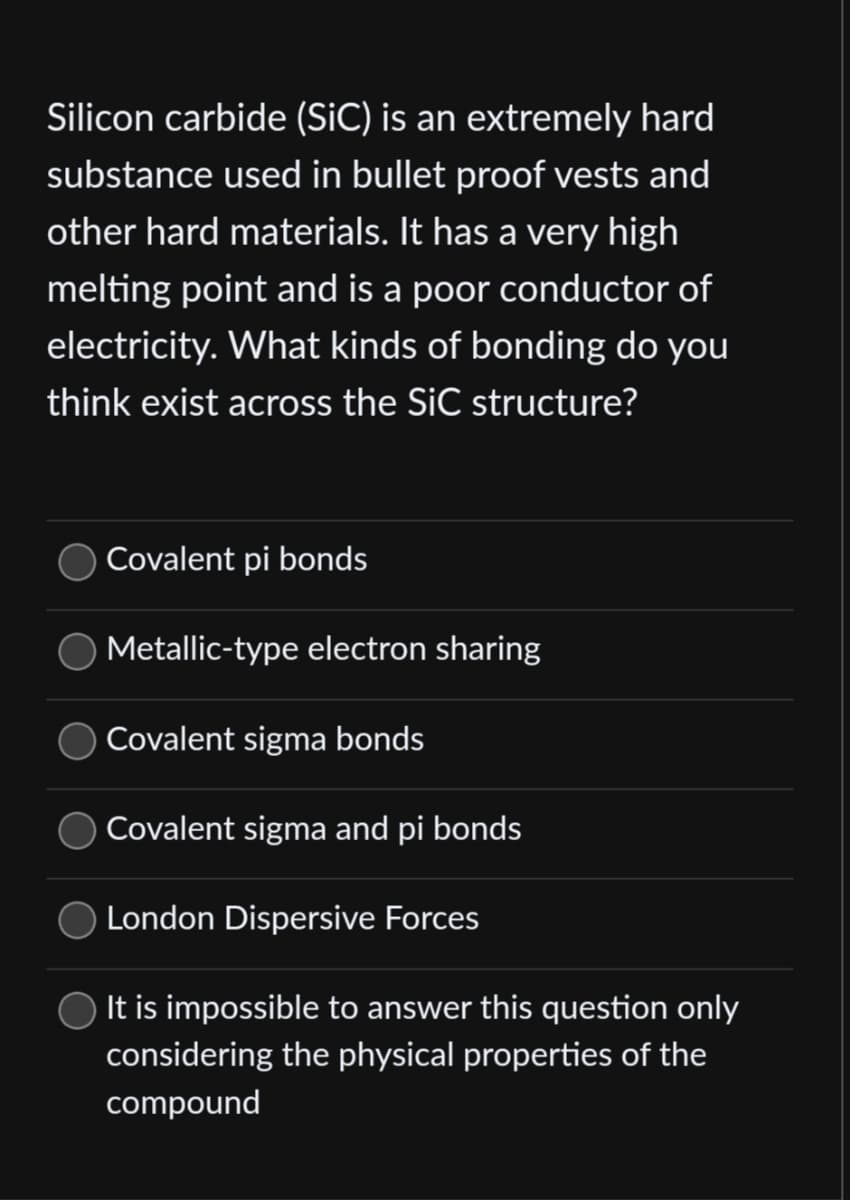
Transcribed Image Text:Silicon carbide (SiC) is an extremely hard
substance used in bullet proof vests and
other hard materials. It has a very high
melting point and is a poor conductor of
electricity. What kinds of bonding do you
think exist across the SiC structure?
Covalent pi bonds
Metallic-type electron sharing
Covalent sigma bonds
Covalent sigma and pi bonds
London Dispersive Forces
It is impossible to answer this question only
considering the physical properties of the
compound
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning