So you escaped the fate of wrapping hamburgers. The next step is to continue proving that you know dimensional analysis (it’s never too late for the hamburger people to come get you!) The great thing about dimensional analysis is that sometimes you can solve problems without equations. Here is such a case: a. All matter has a property called a specific heat capacity. For silver, this specific heat capacity is 0.24 J/°C · g.
So you escaped the fate of wrapping hamburgers. The next step is to continue proving that you know dimensional analysis (it’s never too late for the hamburger people to come get you!) The great thing about dimensional analysis is that sometimes you can solve problems without equations. Here is such a case: a. All matter has a property called a specific heat capacity. For silver, this specific heat capacity is 0.24 J/°C · g.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.94PAE
Related questions
Question
So you escaped the fate of wrapping hamburgers. The next step is to continue proving that
you know dimensional analysis (it’s never too late for the hamburger people to come get you!)
The great thing about dimensional analysis is that sometimes you can solve problems without
equations. Here is such a case:
a. All matter has a property called a specific heat capacity. For silver, this specific heat
capacity is 0.24 J/°C · g. How much energy (in Joules) would be required to heat 120.0 g of
silver (Ag) so that its temperature changes by 32°C? Use dimensional analysis, not an
equation!
you know dimensional analysis (it’s never too late for the hamburger people to come get you!)
The great thing about dimensional analysis is that sometimes you can solve problems without
equations. Here is such a case:
a. All matter has a property called a specific heat capacity. For silver, this specific heat
capacity is 0.24 J/°C · g. How much energy (in Joules) would be required to heat 120.0 g of
silver (Ag) so that its temperature changes by 32°C? Use dimensional analysis, not an
equation!
Dimensional Analysis Practice Problems
Page 6 of 6
b. Based on how you set up the problem above, what would be the equation? Fill in the rest of
this expression to form your own equation (that you figured out by using dimensional analysis
above.) You will use the terms “mass” “specific heat” and “temperature” and some
mathematical operation signs). This answer is an equation, not a dimensional analysis setup!
Page 6 of 6
b. Based on how you set up the problem above, what would be the equation? Fill in the rest of
this expression to form your own equation (that you figured out by using dimensional analysis
above.) You will use the terms “mass” “specific heat” and “temperature” and some
mathematical operation signs). This answer is an equation, not a dimensional analysis setup!
Expert Solution
Step 1
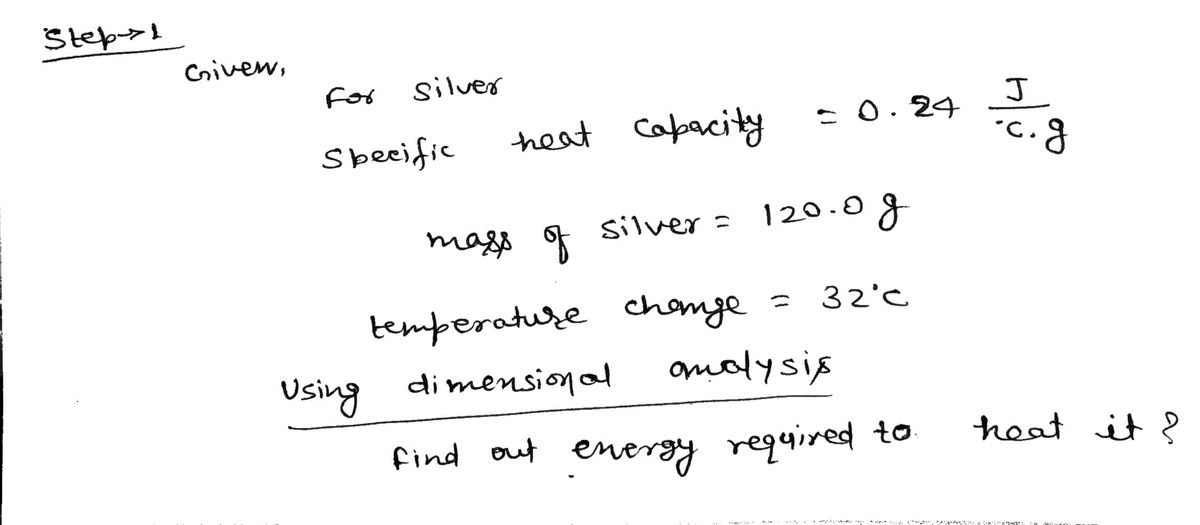
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning