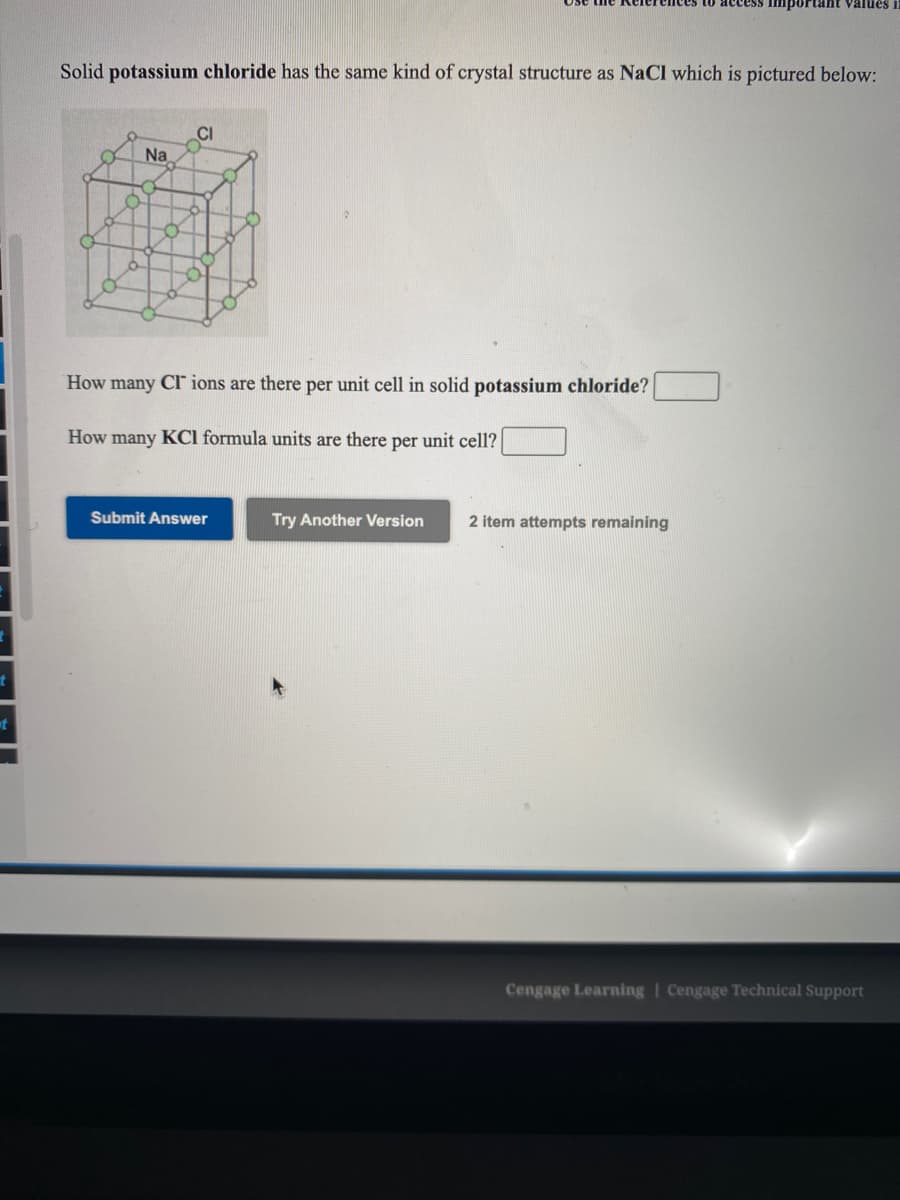
Solid potassium chloride has the same kind of crystal structure as NaCl which is pictured below: CI Na How many CI ions are there per unit cell in solid potassium chloride? How many KCl formula units are there per unit cell?
Solid potassium chloride has the same kind of crystal structure as NaCl which is pictured below: CI Na How many CI ions are there per unit cell in solid potassium chloride? How many KCl formula units are there per unit cell?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 87E: Perovskite is a mineral containing calcium, titanium, and oxygen. Two different representations of...
Related questions
Question
Transcribed Image Text:mportant Välues i
Solid potassium chloride has the same kind of crystal structure as NaCl which is pictured below:
CI
Na
How many Cl ions are there per unit cell in solid potassium chloride?
How many KCl formula units are there per unit cell?
Submit Answer
Try Another Version
2 item attempts remaining
Cengage Learning | Cengage Technical Support
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning