Soluble compounds Almost all salts of Na*, K*, NH,+ Salts of nitrate (NO, ), chlorate (CIO,"), perchlorate (CIO,), acctate (CH,C0,") Almost all salts of CI", Br",I Exceptions (not soluble): halides of Ag*, Hg,, Ph+ Salts containing F Exceptions (not soluble): fluorides of Mg", Ca", Sr, Ba+, Ph* Salts of sulfate (So,) Exceptions (not soluble): sulfates of Ca" , Sr", Ba", Ph", Ag Insoluble compounds Most salts of carbonate (CO, ), phosphate (PO,), oxalate (C20,), chromate (CrO, ), sulfide (S) Exceptions (soluble): salts of NH, * and the alkali metal cations, and Bas Most metal hydroxides and oxides Exceptions (soluble): alkali metal hydroxides and Ba(OH), and Sr(OH), A. Classify each of the compounds as soluble or not soluble: iron(III) acetate zinc nitrate cobalt(II) iodide B. Classify each of the compounds as soluble or not soluble: iron(II) acetate magnesium carbonate silver nitrate C. Classify each of the compounds as soluble or not soluble: ammonium sulfide copper(II) hydroxide cobalt(II) nitrate
Soluble compounds Almost all salts of Na*, K*, NH,+ Salts of nitrate (NO, ), chlorate (CIO,"), perchlorate (CIO,), acctate (CH,C0,") Almost all salts of CI", Br",I Exceptions (not soluble): halides of Ag*, Hg,, Ph+ Salts containing F Exceptions (not soluble): fluorides of Mg", Ca", Sr, Ba+, Ph* Salts of sulfate (So,) Exceptions (not soluble): sulfates of Ca" , Sr", Ba", Ph", Ag Insoluble compounds Most salts of carbonate (CO, ), phosphate (PO,), oxalate (C20,), chromate (CrO, ), sulfide (S) Exceptions (soluble): salts of NH, * and the alkali metal cations, and Bas Most metal hydroxides and oxides Exceptions (soluble): alkali metal hydroxides and Ba(OH), and Sr(OH), A. Classify each of the compounds as soluble or not soluble: iron(III) acetate zinc nitrate cobalt(II) iodide B. Classify each of the compounds as soluble or not soluble: iron(II) acetate magnesium carbonate silver nitrate C. Classify each of the compounds as soluble or not soluble: ammonium sulfide copper(II) hydroxide cobalt(II) nitrate
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 10E: The Handbook of Chemistry and Physics (http://openstaxcollege.org/l/16Handbook) gives solubilities...
Related questions
Question
I need help understanding how to do this homework question. I've been out sick so I don't even know how to even begin to attempt this question.
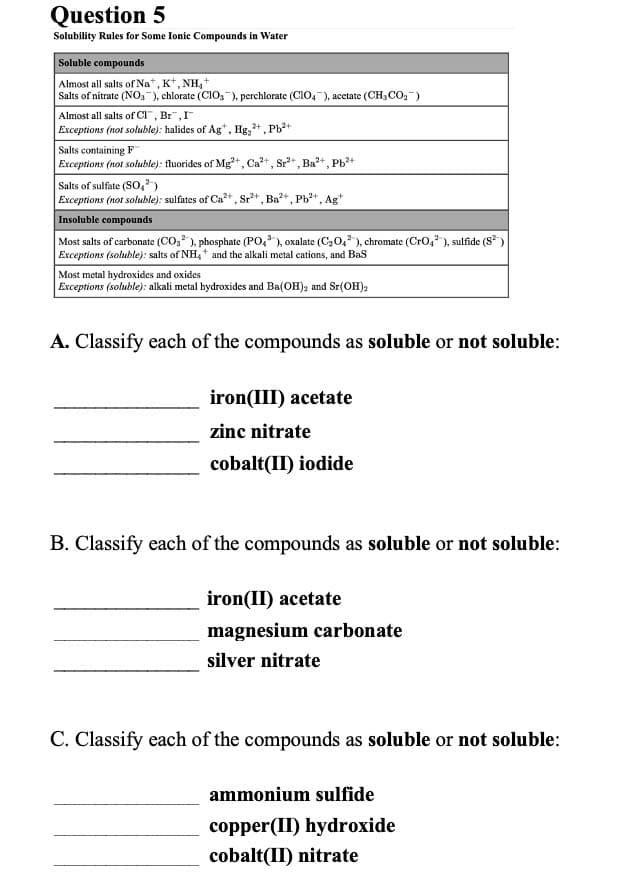
Transcribed Image Text:Question 5
Solubility Rules for Some Ionic Compounds in Water
Soluble compounds
Almost all salts of Nat, K*, NH,+
Salts of nitrate (NO,-), chlorate (CIO,), perchlorate (CIO,-), acetate (CH3 Co,-)
Almost all salts of CI", Br",I
Exceptions (not soluble): halides of Ag*, Hg, +, Pb2+
Salts containing F
Exceptions (not soluble): fluorides of Mg²+, Ca+, Sr+, Ba+, Pb+
Salts of sulfate (So,)
Exceptions (not soluble): sulfates of Ca* , Sr*, Ba+, Pb+, Ag+
Insoluble compounds
Most salts of carbonate (CO, ), phosphate (PO, ), oxalate (C20,), chromate (Cro,"), sulfide (S)
Exceptions (soluble): salts of NH4" and the alkali metal cations, and Bas
Most metal hydroxides and oxides
Exceptions (soluble): alkali metal hydroxides and Ba(OH), and Sr(OH),
A. Classify each of the compounds as soluble or not soluble:
iron(III) acetate
zinc nitrate
cobalt(II) iodide
B. Classify each of the compounds as soluble or not soluble:
iron(II) acetate
magnesium carbonate
silver nitrate
C. Classify each of the compounds as soluble or not soluble:
ammonium sulfide
copper(II) hydroxide
cobalt(II) nitrate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax