Sulphur reacts with carbon and potassium separately to form compound P and compound Q. (a) Draw an electron diagram of compound P, showing electrons in the outermost shells only. (b) (i) Draw an electron diagram of compound Q, showing electrons in the outermost shells only. (ii) Discuss, with explanation, the electrical conductivity of compound Q with reference to the type and property of the particles in it. Both fluorine, chlorine and bromine are Group VII elements in the periodic table. (a) To which period of the periodic table does bromine belong? Explain your answer. (b) Draw an electron diagram of the compound formed between fluorine and chlorine, showing electrons in the outermost shells only. (c) Sodium reacts with chlorine to form sodium chloride. (0) Finish the following diagram to show the structure of sodium chloride. (ii) Explain why solid sodium chloride is brittle.
Sulphur reacts with carbon and potassium separately to form compound P and compound Q. (a) Draw an electron diagram of compound P, showing electrons in the outermost shells only. (b) (i) Draw an electron diagram of compound Q, showing electrons in the outermost shells only. (ii) Discuss, with explanation, the electrical conductivity of compound Q with reference to the type and property of the particles in it. Both fluorine, chlorine and bromine are Group VII elements in the periodic table. (a) To which period of the periodic table does bromine belong? Explain your answer. (b) Draw an electron diagram of the compound formed between fluorine and chlorine, showing electrons in the outermost shells only. (c) Sodium reacts with chlorine to form sodium chloride. (0) Finish the following diagram to show the structure of sodium chloride. (ii) Explain why solid sodium chloride is brittle.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 86AP
Related questions
Question
Hello, may someone help me thankyou!! urgent!
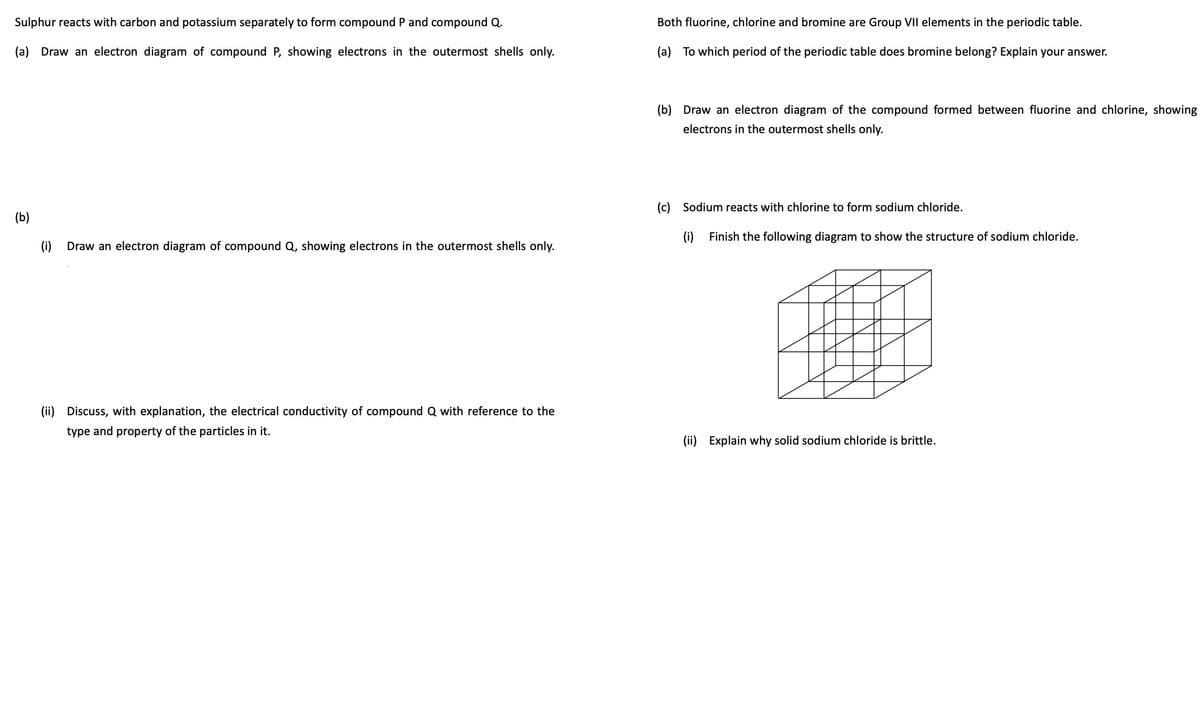
Transcribed Image Text:Sulphur reacts with carbon and potassium separately to form compound P and compound Q.
(a) Draw an electron diagram of compound P, showing electrons in the outermost shells only.
(b)
(i) Draw an electron diagram of compound Q, showing electrons in the outermost shells only.
(ii) Discuss, with explanation, the electrical conductivity of compound Q with reference to the
type and property of the particles in it.
Both fluorine, chlorine and bromine are Group VII elements in the periodic table.
(a) To which period of the periodic table does bromine belong? Explain your answer.
(b) Draw an electron diagram of the compound formed between fluorine and chlorine, showing
electrons in the outermost shells only.
(c) Sodium reacts with chlorine to form sodium chloride.
(i)
Finish the following diagram to show the structure of sodium chloride.
(ii) Explain why solid sodium chloride is brittle.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning