Table 2: Molarity of H2O2 and KI and Reaction Rate Trial H2O2 Concentration, M KI Concentration, M Reaction Rate (Reciprocal Slope) 1 0.29 M 0.40 M 14.08 2 0.29 M 0.20M 25 3 0.023 M 0.40 M 20 please help me with this part Rate constant value Trial 1 ______________ Trial 2 ______________
Table 2: Molarity of H2O2 and KI and Reaction Rate Trial H2O2 Concentration, M KI Concentration, M Reaction Rate (Reciprocal Slope) 1 0.29 M 0.40 M 14.08 2 0.29 M 0.20M 25 3 0.023 M 0.40 M 20 please help me with this part Rate constant value Trial 1 ______________ Trial 2 ______________
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.13QAP
Related questions
Question
Table 2: Molarity of H2O2 and KI and Reaction Rate
|
Trial |
H2O2 Concentration, M |
KI Concentration, M |
Reaction Rate |
|
1 |
0.29 M |
0.40 M |
14.08 |
|
2 |
0.29 M |
0.20M |
25 |
|
3 |
0.023 M |
0.40 M |
20 |
please help me with this part
Rate constant value
Trial 1 ______________
Trial 2 ______________
Trial 3 ______________
Average _____________
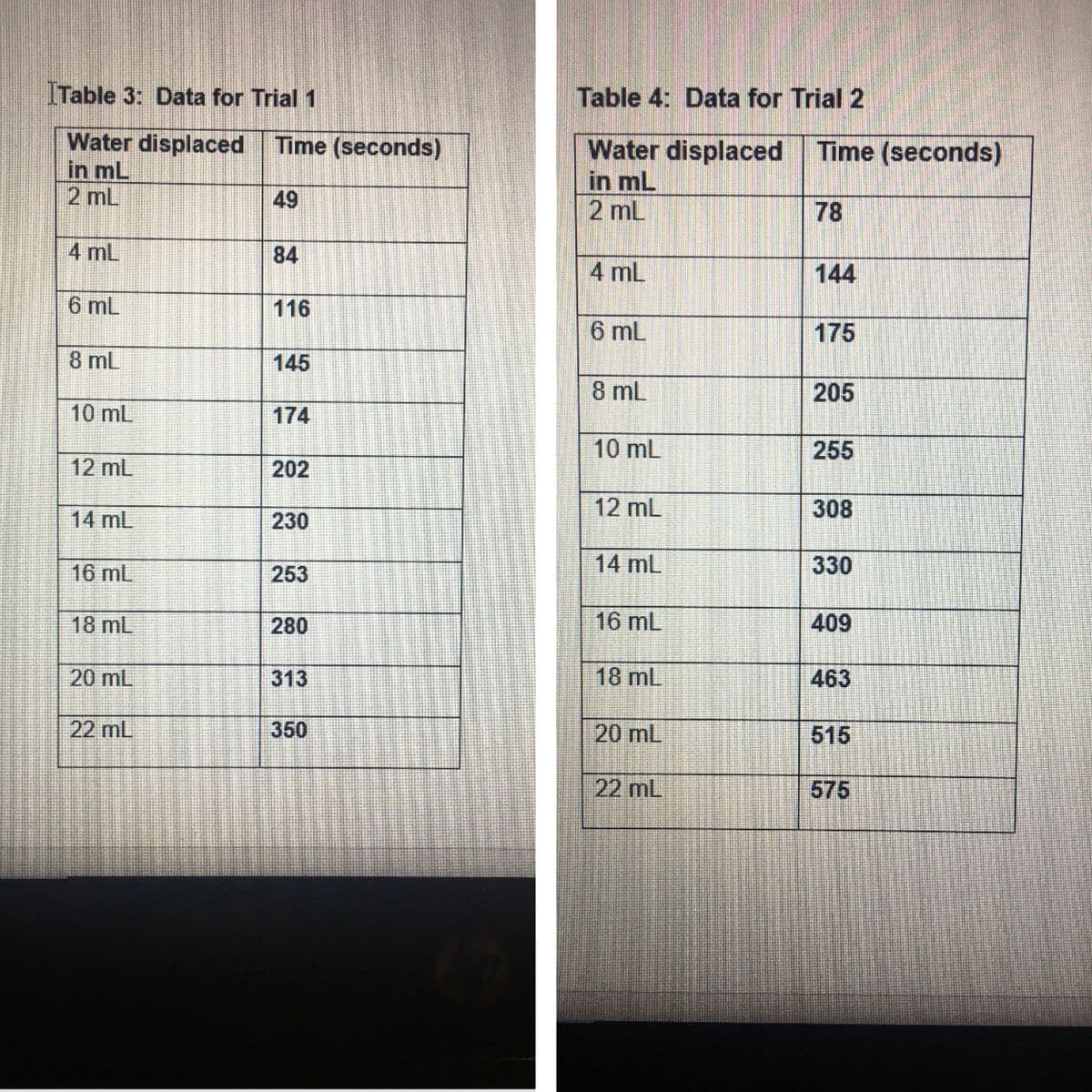
Transcribed Image Text:ITable 3: Data for Trial 1
Table 4: Data for Trial 2
Water displaced
in mL
Time (seconds)
Water displaced
in mL
2 mL
Time (seconds)
2 mL
49
78
4 mL
84
4 mL
144
6 mL
116
6 mL
175
8 mL
145
8 mL
205
10 mL
174
10 mL
255
12 mL
202
12 mL
308
14 mL
230
16 mL
253
14 mL
330
18 mL
280
16 mL
409
20 mL
313
18 mL
463
22 mL
350
20 mL
515
22 mL
575
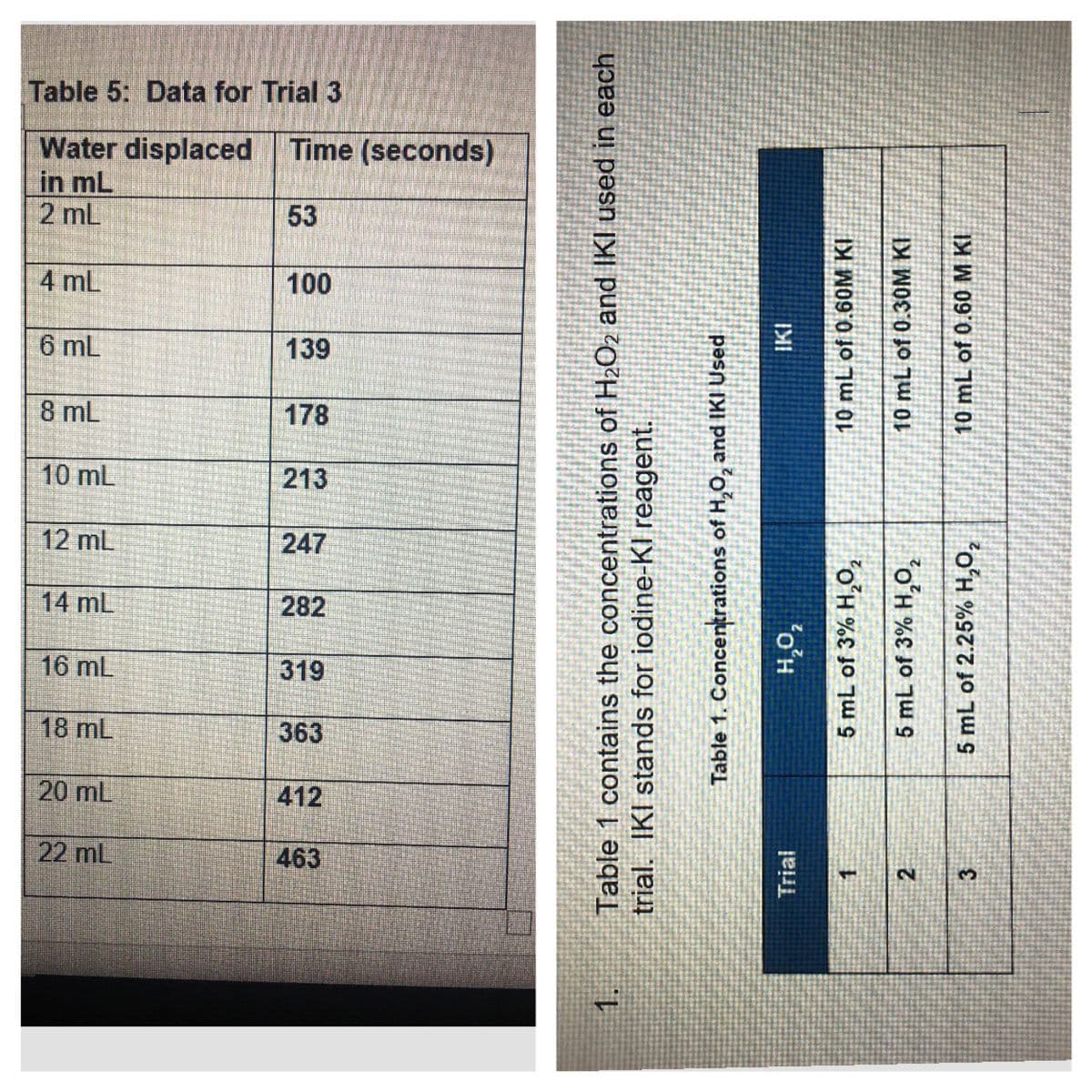
Transcribed Image Text:Table 5: Data for Trial 3
Water displaced Time (seconds)
in mL
2 mL
53
4 mL
100
6 mL
139
8 mL
178
10 mL
213
12 mL
247
14 mL
282
319
20 mL
22 mL
2.
9.
Table 1 contains the concentrations of H2O2 and IKI used in each
trial. IKI stands for iodine-KI reagent.
Table 1. Concentrations of H,O, and IKI Used
Trial
5 mL of 3% H,0,
10 mL of 0.60M KI
5 mL of 3% H,O,
10 mL of 0.30M KI
5 mL of 2.25% H,O,
10 mL of 0.60 M KI
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole