Data Table: Trial 2 (seconds) Trial 1 (seconds) 11.29-6895ccande 1.09M=6096cc0nds Whole Crushed 3.16M-195600omde 315M=1955ecands Whole 6795econds Average whole:679scconds /Check out: Question: What is the effect of particle size on the reaction rate? Claim The whole Particie Si ze have the More surfface area Evidence Reasoning
Data Table: Trial 2 (seconds) Trial 1 (seconds) 11.29-6895ccande 1.09M=6096cc0nds Whole Crushed 3.16M-195600omde 315M=1955ecands Whole 6795econds Average whole:679scconds /Check out: Question: What is the effect of particle size on the reaction rate? Claim The whole Particie Si ze have the More surfface area Evidence Reasoning
Chapter30: Kinetic Methods Of Analysis
Section: Chapter Questions
Problem 30.10QAP
Related questions
Question
Explain what is the effect of particle size on the reaction rate with explanation , consider the relationship between the particle size , surface area , atoms ability to collide.
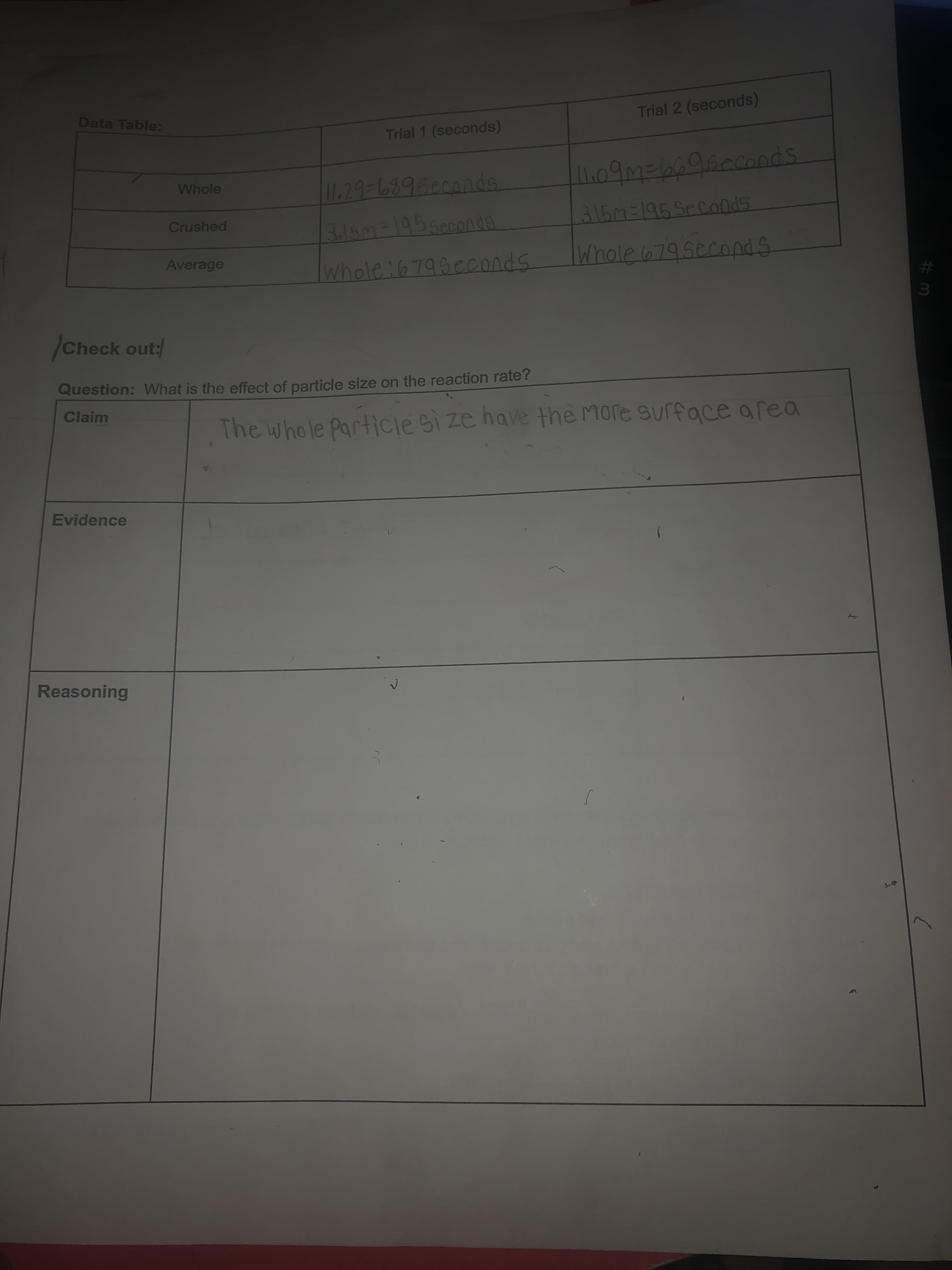
Transcribed Image Text:Data Table:
Trial 2 (seconds)
Trial 1 (seconds)
09M=66960conds
Whole
1179-68950c0nds
Crushed
316m-195400onda
Whole 6795econdS
Average
whole:6799cconds
%23
3.
Check out
Question: What is the effect of particle size on the reaction rate?
Claim
The whole Particie Si ze have the More surface area
Evidence
Reasoning
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,