Table 11.3 (data) -cm les 10-cm Test Tube 15-cm Test Tube nount of KIO3 ount of NaHSO ria Number mount of Water 3Reaction Temp 20 drops 20 drops 20 drops 30 drops 30 drops 30 drops 10 drops 20 C 10 drops 15 C 10 drops 35 C Reaction Time 0:36 1:13 0:18 2 3 Table 11.4 (report) rial Number Temperature eaction Rate eac an 2 for 10°C decrease for 10°C increase 3
Table 11.3 (data) -cm les 10-cm Test Tube 15-cm Test Tube nount of KIO3 ount of NaHSO ria Number mount of Water 3Reaction Temp 20 drops 20 drops 20 drops 30 drops 30 drops 30 drops 10 drops 20 C 10 drops 15 C 10 drops 35 C Reaction Time 0:36 1:13 0:18 2 3 Table 11.4 (report) rial Number Temperature eaction Rate eac an 2 for 10°C decrease for 10°C increase 3
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 20E: The density of liquid NH3 is 0.64 g/mL; the density of gaseous NH3 at STP is 0.0007 g/mL. Explain...
Related questions
Question
This is possible because of the following reactions that take place rapidly as long as reactants are available:
(11.3)
Equation : 51-(aq) + IO-3(aq) + 6H+(aq) ----> 3I2(aq) +3H2O(I)
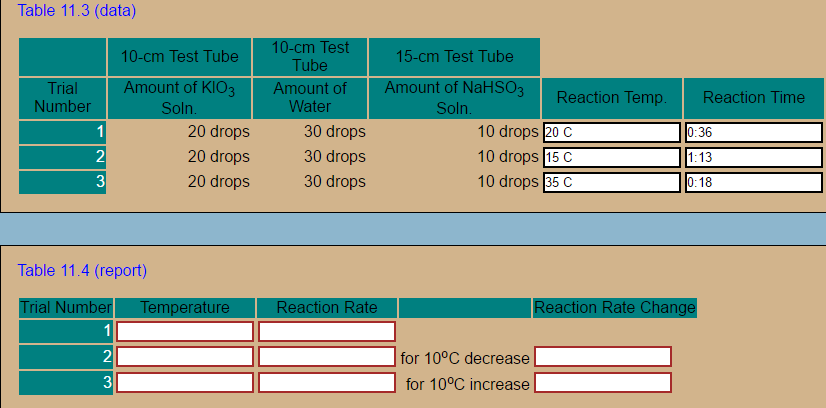
Transcribed Image Text:Table 11.3 (data)
-cm les
10-cm Test Tube
15-cm Test Tube
nount of KIO3
ount of NaHSO
ria
Number
mount of
Water
3Reaction Temp
20 drops
20 drops
20 drops
30 drops
30 drops
30 drops
10 drops 20 C
10 drops 15 C
10 drops 35 C
Reaction Time
0:36
1:13
0:18
2
3
Table 11.4 (report)
rial Number
Temperature
eaction Rate
eac
an
2
for 10°C decrease
for 10°C increase
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning