The activity of penicillin is proportional to its concentration. This antibiotic decomposes slowly when stored in pH 7 at room temperature. The time dependence of this decomposition is given below. Does this decomposition follow first or second order kinetics? (50 points) sone = Act x m Time (weeks) Activity 10100 8180 6900 5380 3870 8. 2000 10 1330 12 898 15 403
The activity of penicillin is proportional to its concentration. This antibiotic decomposes slowly when stored in pH 7 at room temperature. The time dependence of this decomposition is given below. Does this decomposition follow first or second order kinetics? (50 points) sone = Act x m Time (weeks) Activity 10100 8180 6900 5380 3870 8. 2000 10 1330 12 898 15 403
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter18: Chemical Kinetics
Section: Chapter Questions
Problem 49P
Related questions
Question
100%
Question attached as photo.
This is not a exam/ test question:) you can see that our prof has written sth on this question, it is a question that he wants us to practice on our own in lecture. Thanks
Topic related to 1st order/ 2nd order kinetics, need excel/graph to do this question, need TWO plots to check whether they are 1st/2nd order
concentration = activity * m (concentration is proportional to activity plus a constant m)
1/[A] =1/[A]0 + Kt
1/[Activity* m] = 1/[Activity0 *m] +Kt
1/[Activity] =1/ [Activity]0 + mKt
- Do we follow the equations of zeroth, 1st, 2nd order reactions?
- What is the key idea to determine whether this data gives 1st or 2nd order kinetics?
- Our prof was saying that if it's linear graph, means it is in correct order. BUT i have plotted 3 datas, two of them looks kinda linear to me. (the 2nd order and zeroth order graph are similar) If we are choosing from zeroth/2nd order, how do we determine its order if it's line is similar in linearity?
![1/Activity
0.006
Time (weeks)
In[Activity]- 1st order
9.22029070282935
0.005
1
9.00944742959679
2
8.83927669058535
0.003
8.59044365315583
8.26100978602383
0.002
7.60090245954208
10
7.1929342212158
1
2
3
8 10 12 15 20
12
6.8001700683022
Time (weeks)
1/ Activity - 2nd order
15
5.99893656194668
0.000099009900990099
20
5.11799381241676
1
0.000122249388753056
- Untitled 1
0.000144927536231884
0.000185873605947955
11000
0.000258397932816537
8250
8
0.0005
5500
10
0.00075187969924812
12
0.00111358574610245
2750
15
0.00248138957816377
1.
2
3
8
10 12 15 20
20
0.00591715976331361
- In Activity
Time (weeks)
Activity - zeroth order
10
10100
1
8180
7.5
2
6900
5380
3870
8
2000
2.5
10
1330
12
898
0 1
2
3
5
8
10
12
15
20
LO](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fe0dd0615-5392-46ed-af9f-2afbe8658b62%2Fb069af43-7597-4334-9aa8-8f3c2c8a5d48%2Fuw7ov6y_processed.png&w=3840&q=75)
Transcribed Image Text:1/Activity
0.006
Time (weeks)
In[Activity]- 1st order
9.22029070282935
0.005
1
9.00944742959679
2
8.83927669058535
0.003
8.59044365315583
8.26100978602383
0.002
7.60090245954208
10
7.1929342212158
1
2
3
8 10 12 15 20
12
6.8001700683022
Time (weeks)
1/ Activity - 2nd order
15
5.99893656194668
0.000099009900990099
20
5.11799381241676
1
0.000122249388753056
- Untitled 1
0.000144927536231884
0.000185873605947955
11000
0.000258397932816537
8250
8
0.0005
5500
10
0.00075187969924812
12
0.00111358574610245
2750
15
0.00248138957816377
1.
2
3
8
10 12 15 20
20
0.00591715976331361
- In Activity
Time (weeks)
Activity - zeroth order
10
10100
1
8180
7.5
2
6900
5380
3870
8
2000
2.5
10
1330
12
898
0 1
2
3
5
8
10
12
15
20
LO
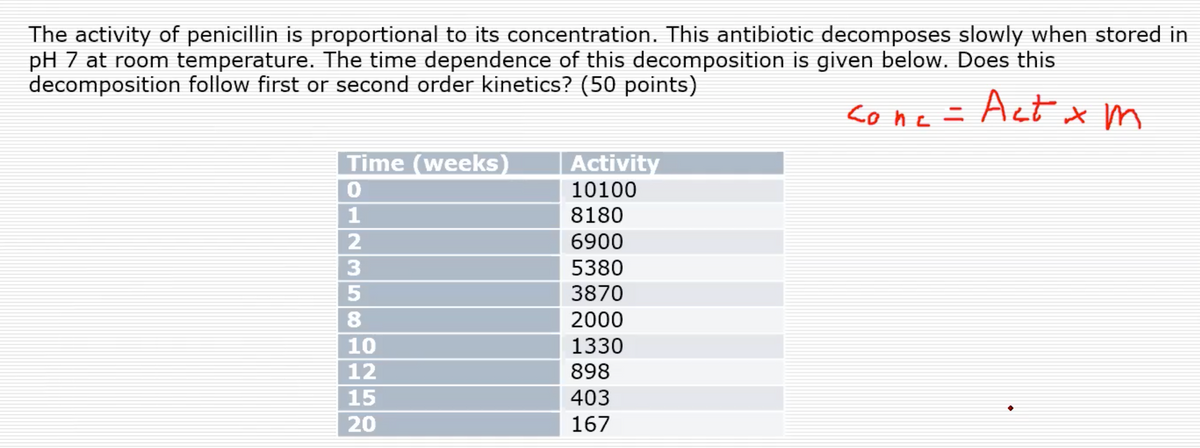
Transcribed Image Text:The activity of penicillin is proportional to its concentration. This antibiotic decomposes slowly when stored in
pH 7 at room temperature. The time dependence of this decomposition is given below. Does this
decomposition follow first or second order kinetics? (50 points)
cone= Act x m
Act
Time (weeks)
Activity
10100
8180
6900
5380
3870
8.
2000
10
1330
12
898
15
403
20
167
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning