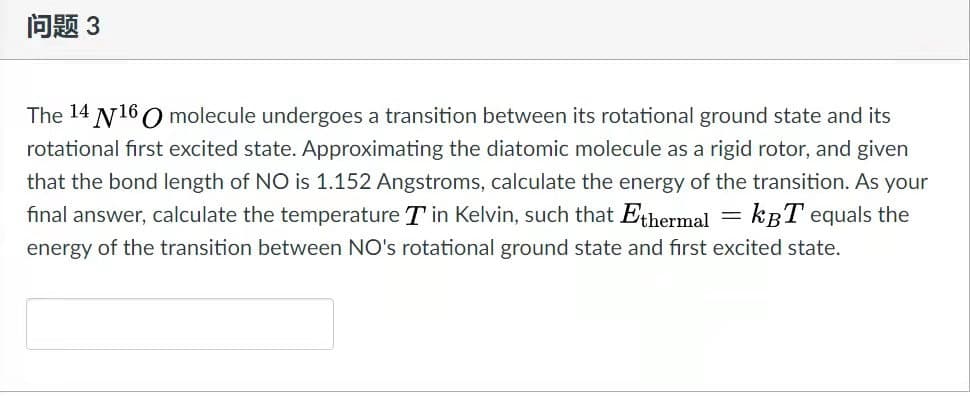
The 14 N160 molecule undergoes a transition between its rotational ground state and its rotational first excited state. Approximating the diatomic molecule as a rigid rotor, and given that the bond length of NO is 1.152 Angstroms, calculate the energy of the transition. As your final answer, calculate the temperature T in Kelvin, such that Ethermal = kBT equals the energy of the transition between NO's rotational ground state and fırst excited state.
The 14 N160 molecule undergoes a transition between its rotational ground state and its rotational first excited state. Approximating the diatomic molecule as a rigid rotor, and given that the bond length of NO is 1.152 Angstroms, calculate the energy of the transition. As your final answer, calculate the temperature T in Kelvin, such that Ethermal = kBT equals the energy of the transition between NO's rotational ground state and fırst excited state.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 51AP
Related questions
Question
Transcribed Image Text:问题3
The 14 N160 molecule undergoes a transition between its rotational ground state and its
rotational first excited state. Approximating the diatomic molecule as a rigid rotor, and given
that the bond length of NO is 1.152 Angstroms, calculate the energy of the transition. As your
final answer, calculate the temperature T in Kelvin, such that Eshermal = kBT equals the
energy of the transition between NO's rotational ground state and first excited state.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning