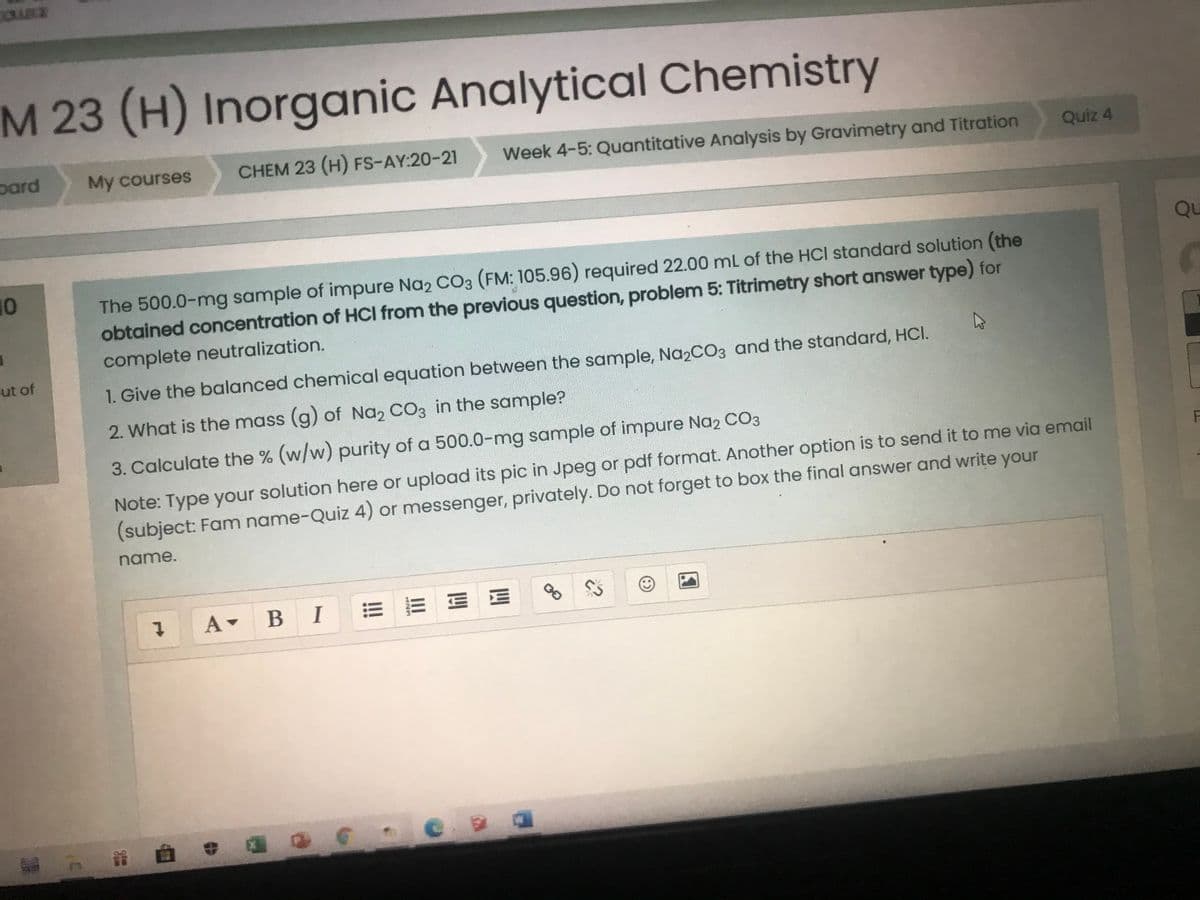
The 500.0-mg sample of impure Na, CO3 (FM: 105.96) required 22.00 mL of the HCI standard solution (the obtained concentration of HCI from the previous question, problem 5: Titrimetry short answer type) for complete neutralization. 1. Give the balanced chemical equation between the sample, Na2CO3 and the standard, HCI. 2. What is the mass (g) of Na, CO3 in the sample? 3. Calculate the % (w/w) purity of a 500.0-mg sample of impure Na2 CO3 Note: Type your solution here or upload its pic in Jpeg or pdf format. Another option is to send it to me via email (subject: Fam name-Quiz 4) or messenger, privately. Do not forget to box the final answer and write your name.
The 500.0-mg sample of impure Na, CO3 (FM: 105.96) required 22.00 mL of the HCI standard solution (the obtained concentration of HCI from the previous question, problem 5: Titrimetry short answer type) for complete neutralization. 1. Give the balanced chemical equation between the sample, Na2CO3 and the standard, HCI. 2. What is the mass (g) of Na, CO3 in the sample? 3. Calculate the % (w/w) purity of a 500.0-mg sample of impure Na2 CO3 Note: Type your solution here or upload its pic in Jpeg or pdf format. Another option is to send it to me via email (subject: Fam name-Quiz 4) or messenger, privately. Do not forget to box the final answer and write your name.
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.40QAP
Related questions
Question
Transcribed Image Text:M 23 (H) Inorganic Analytical Chemistry
pard
My courses
CHEM 23 (H) FS-AY:20-21
Week 4-5: Quantitative Analysis by Gravimetry and Titration
Quiz 4
Qu
10
The 500.0-mg sample of impure Na2 CO3 (FM: 105.96) required 22.00 mL of the HCI standard solution (the
obtained concentration of HCI from the previous question, problem 5: Titrimetry short answer type) for
complete neutralization.
ut of
1. Give the balanced chemical equation between the sample, Na2CO3 and the standard, HCI.
2. What is the mass (g) of Na, CO3 in the sample?
3. Calculate the % (w/w) purity of a 500.0-mg sample of impure Na2 CO3
Note: Type your solution here or upload its pic in Jpeg or pdf format. Another option is to send it to me via email
(subject: Fam name-Quiz 4) or messenger, privately. Do not forget to box the final answer and write your
name.
В I
1.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
