Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter12: States Of Matter
Section: Chapter Questions
Problem 70A
Related questions
Question
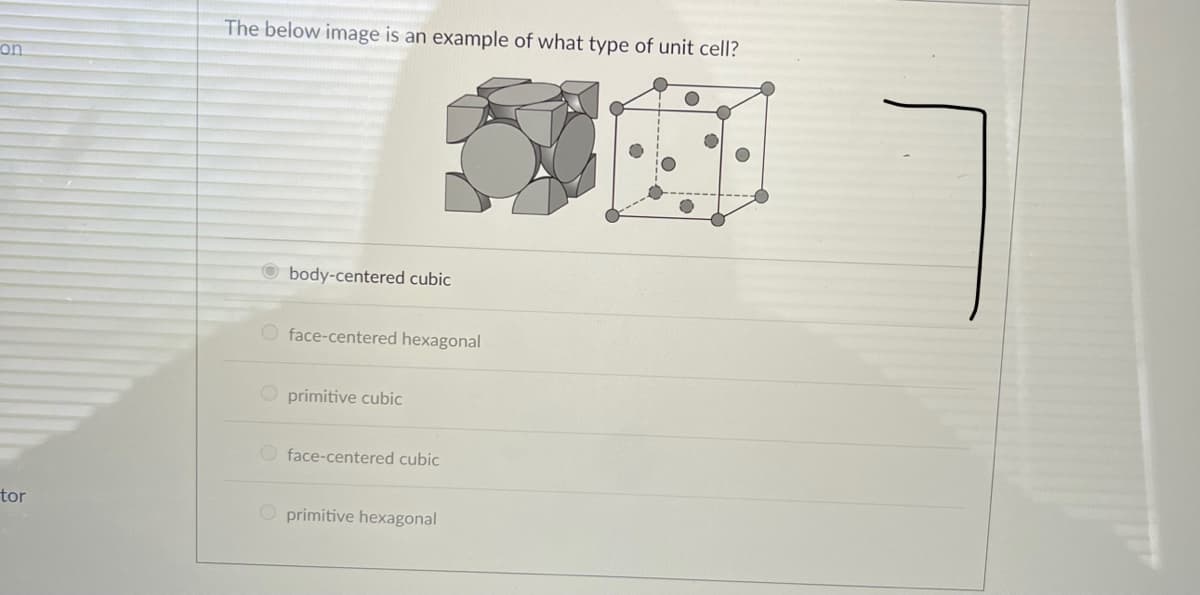
Transcribed Image Text:The below image is an example of what type of unit cell?
on
O body-centered cubic
O face-centered hexagonal
O primitive cubic
O face-centered cubic
tor
O primitive hexagonal
![In a pure metal, the electrons can be thought of as concentrated around atoms
throughout the metal. Using molecular orbital theory, there
[ Select ]
an energy gap between the filled molecular orbitals
ion
and empty molecular orbitals. The [Select ]
orbitals are
typically higher in energy and are mostly [Select ]
Answer 1:
concentrated around atoms
Answer 2:
is
Answer 3:
antibonding
ctor
Answer 4:
filled](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F9e9d24fe-7c27-4389-9274-003601dc9bbe%2F03b419e0-715f-4244-a483-f5432367b423%2Fiwle4pi_processed.jpeg&w=3840&q=75)
Transcribed Image Text:In a pure metal, the electrons can be thought of as concentrated around atoms
throughout the metal. Using molecular orbital theory, there
[ Select ]
an energy gap between the filled molecular orbitals
ion
and empty molecular orbitals. The [Select ]
orbitals are
typically higher in energy and are mostly [Select ]
Answer 1:
concentrated around atoms
Answer 2:
is
Answer 3:
antibonding
ctor
Answer 4:
filled
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,