The compound dimethylamine is a weak base like ammonia. A solution contains 0.316 M (CH),NH,* and 0.117 M dimethylamine, (CH3),NH. The pH of this solution is Submit Answer Retry Entire Group 9 more group attempts remaining
The compound dimethylamine is a weak base like ammonia. A solution contains 0.316 M (CH),NH,* and 0.117 M dimethylamine, (CH3),NH. The pH of this solution is Submit Answer Retry Entire Group 9 more group attempts remaining
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 36P
Related questions
Question
Show your all calculation
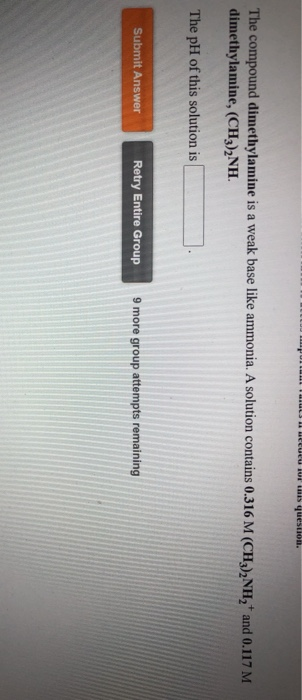
Transcribed Image Text:The compound dimethylamine is a weak base like ammonia. A solution contains 0.316 M (CH),NH,* and 0.117 M
dimethylamine, (CH3),NH.
The pH of this solution is
Submit Answer
Retry Entire Group
9 more group attempts remaining
Expert Solution
Step 1
The given solution is a buffer mixture of weak base dimethylamine, (CH3)2NH and conjugate acid (CH3)2NH2+. Thus, we can use Henderson - Hasselbalch equation to calculate the pOH and subsequently the pH of the solution. It is given by,
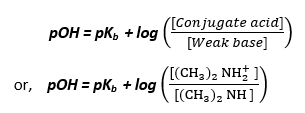
We know that, Kb for (CH3)2NH = 5.40 x 10-4
Thus, pKb = -log Kb = - log( 5.40 x 10-4) = 3.27
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

