Q: 1.35mol of copper reacts with an excess of silver nitrate according to the reaction above. from this…
A:
Q: The Kc of the reaction below is 3.149x10 -4 at 351.0K. What is the Kp value for this reaction. The…
A: The relationship between Kp and Kc is => Kp = Kc (RT)Δn where, T is the temperature in kelvin,…
Q: The boundary and energy diagrams for the valence atomic orbitals before examining bonding theories.…
A:
Q: X = Cl or l Select one alternative: Olodocyclohexane is more stable than chlorocyclohexane The pKaH…
A: Answer: E1 elimination reaction takes place through carbocation formation and since carbocation is…
Q: You are using a Vernier Colorimeter to measure the concentration of Yellow dye #6 in Powerade Which…
A: Yellow dye #6 has a maximum absorption at around 430 nm, which means that it will absorb light most…
Q: What mass of silver would plate onto the cathode if a current of 6.7 AA flowed through the cell for…
A:
Q: 1) What is the molarity of a HCI solution if 28.3 mL of HCl is required to react with 0.256 g of…
A: Dear student since you have posted multiple questions,As per BARTLEBY QnA guidelines We are allowed…
Q: Is it 3.52?
A: Yes it is 3.52 Graph is
Q: Give detailed Solution with explanation needed..complete the following reaction
A: We have been given an organic reaction and we have been asked to complete given organic reaction.…
Q: Draw the products of the reaction shown below. Ignore inorganic byproducts. NO₂ cat. H₂SO4 HNO, (1…
A:
Q: 4. Predict the product of the following reaction. i i AICI3, then H₂O
A: Given that, a reaction scheme is shown below We have to predict the product of the above reaction.…
Q: The value of Kc for the reaction below is 0.210 at 373 K. N2O4 (g) ⇌ 2 NO2 (g) a. Mathematically…
A: Given data ; N2O4(g) <==> 2 NO2(g) Kc = 0.210 initial concentration of N2O4 = 0.300 M
Q: For 2.277g of calcium chloride, the mass of calcium by itself is 0.825g. What is the mass of…
A: Given, mass of Calcium chloride (CaCl2) = 2.277 g mass of Calcium (Ca) = 0.825 g mass of chlorine…
Q: A chemistry graduate student is given 300. mL of a 1.20M methylamine (CH3NH₂) solution. Methylamine…
A: Given: Volume of solution = 300 mLMolarity of CH3NH2 = 1.20 MpH of solution = 10.16Kb of CH3NH2 =…
Q: Calculate the initial temperature, in °C, for a 111-g sample of magnesium (s = 1.02 J/gK) that is…
A:
Q: At a certain temperature the rate of this reaction is first order in CICH₂CH₂Cl with a rate constant…
A: Knowing the rate constant, can help us in knowing the rate of decomposition of the starting…
Q: When CH4(g) reacts with O2(g) to form CO₂(g) and H₂O(g), 802 kJ of energy are evolved for each mole…
A: Balanced chemical equation: Balanced chemical equation can be define as the reaction in which number…
Q: Organic chemistry: Write a pair of insoluble, soluble, and miscible solvent.
A: Solubility is the ability of a substance (the solute) to dissolve in another substance (the solvent)…
Q: Draw a structural formula for the major ionic form of the amino acid shown below when in aqueous…
A: we have to draw the structure of amino acid at pH = 1.5
Q: explain in great detail why the pKa values are what they are and compare them among the three…
A: Basicity is defined as electron donation ability of a molecule. If it is able to donate electrons…
Q: What happens to the reaction below when B is removed from the reaction mixture? A (g) + 3B (g) ⇌ 4C…
A:
Q: Consider the following reaction: CoCl₂(9) CO(g) + Cl₂(9) If 3.05x103 moles of COCI₂, 0.203 moles of…
A: Answer: Value of equilibrium constant KC is equal to the ratio of molar concentration of products…
Q: 0:08 AM Thu Mar 23 Problem 33 of 23 Br2 (1 equiv) Draw the product of the reaction shown below.…
A:
Q: Mg(s) + 2HCl(aq) → MgCl2 + H2(g) You will measure the rate of the reaction using a continuous…
A: Rate of reaction can be obtained by monitoring how fast/slow a reactant is being used up or how fast…
Q: 20. Teflon is the polymer used to create non-stick surfaces on cookware. The monomer for Teflon is…
A: Since you have posted a question with multiple sub-parts ,we will solve first three sub-parts for…
Q: Ph Ph A H Ph H Ph Ph Ph B OH Me Me Ph HBr / H₂O Ph Br C Me Ph ? Ph D Br Me Ph Ph E H
A: We know E1 reaction involves first removal of leaving group followed by removal of H- .
Q: What is the final concentration of a solution if 113-mL of a 3.9-M solution was diluted to 406-mL?
A:
Q: For the equilibrium CH4(g) + H₂O(g) CO(g) + 3H2(g) AH = 206.2 kJ select all that apply. Compressing…
A: Answer: This question is based Le-chatalier's principle which states that on changing any parameter…
Q: Draw the structural and line bond formula. Explain. 4-ethyl-2 hexene
A: We have to draw the structural and line bond formula of 4-ethyl-2 hexene.
Q: If an average sodium atom weighs 22.99 amu, how many sodium atoms are contained in 6.05 x 1013 amu…
A: We will use following formula-
Q: A chemistry graduate student is given 300. ml. of a 0.60M pyridine (C,H,N) solution. Pyridine is a…
A:
Q: What is the normality of a 4-L solution of 50.12 g sulfuric acid (MW=98.0 g)? a 0.13 N b None of…
A: Given Volume of solution= 4 litres Mass of sulphuric acid = 50.12 gm Molecular weight= 98 gm
Q: Provide names for the following five saturated hydrocarbons. a) (c) it m-e-m H-C-Ċ-Ċ- I--I H-C-H { H…
A: IUPAC nomenclature is a system of naming chemical compounds in a standardized and systematic way.…
Q: A certain reaction is second order in N₂ and first order in H₂. Use this information to complete the…
A:
Q: A buffer containing acetic acid and sodium acetate has a pH of 5.55. The Ka value for CH3CO2H is…
A: We know PKa = -logka and PH = PKa + log[salt]/[acid]
Q: What is the concentration of salicylate in mg/dL in an unknown salicylate solution whose absorbance…
A: Graph is
Q: NO is weaker or stronger pi-acceptor ligands
A: Both NO and CO are pi-acceptor ligands, meaning they can accept electron density from the metal…
Q: Study this chemical reaction: Pb+21₂ → PbI4 - Then, write balanced half-reactions describing the…
A: Oxidation is the chemical reaction in which the substrate loses electrons. Reduction is the chemical…
Q: The following experimental data was collected by a student: 0.0020 M Fe(NO3)3 mL Trial 1234 Blank…
A: Given :- . Kc = ? -------------------------------------
Q: Consider the reaction of 10 g KClO3 and 5g P4. Identify the limiting reagent Identify the excess…
A: Limiting reagent : Limiting reagents are those reagents that are consumed completely in a chemical…
Q: What Al(III) species is the major species in aqueous solution at pH 14.0 ? a) [Al(H2O)6]3+ b)…
A: pH stands for "potential of hydrogen" and is a measure of the acidity or alkalinity of a solution. A…
Q: Which of the following describes the change in entropy? Select the correct answer below: O O O grev…
A: Entropy is a thermodynamic property that measures the degree of randomness or disorder in a system.…
Q: Write the correct IUPAC name for the following stucture her
A: IUPAC nomenclature is used for naming the organic compound as recommended by international union of…
Q: Correlate the structures of the standard food dyes to their relative position in the paper…
A: "Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: How are the compounds in each pair related to each other? Are they identical, enantiomers,…
A: Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: Consider the weak acid, HA, in water as shown in the reaction below. The [HA] is 0.430 M and the k a…
A:
Q: 1. a. Estimate the % uncertainty in your measurement of: mass of water mass of metal AT of water…
A: We need to find out the percentage uncertainty in the measurements. % Uncertainty of measurement =…
Q: for density is M/\ Calculate the molarity of solution prepared by weighing 6.34 g of NaOH using a…
A:
Q: A solution is prepared at 25 °C that is initially 0.42M in trimethylamine ((CH3),N), ((CH3)NHC1).…
A:
Q: Please answer fast it’s very important and urgent I say very urgent so please answer super super…
A: Solutions- Percentage uncertainty in the measurements.
need the rate constant, half life, lifetime and how long for all ammonia to be consumed
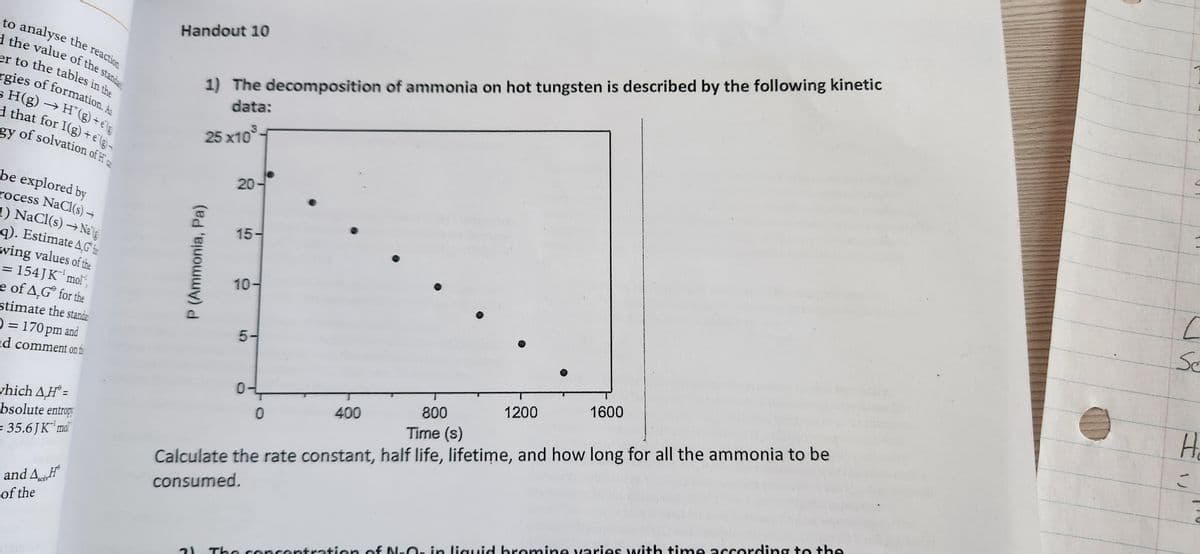
Step by step
Solved in 6 steps with 2 images

- For the dissolution of LiCl in water, ΔHsoln = -37 kJ/mol.Which term would you expect to be the largest negativenumber: ΔHsolvent, ΔHsolute, or ΔHmix?Table 2. Gibbs Free Energies of formation (kJ), ∆G°f, for Ions in 1M Solution and Ionic Solids cation anions Cl- -131.228 I- -51.57 NO3- -108.74 SO4-2 -744.53 Na+ -261.905 -384.138 -286.06 -367.00 -3646.85 W10 ∆G°f of water = -237.129 kJ/mol Calculated values of ∆G°rxn and the ∆Grxn of each box, Predicted results (ppt or no ppt). Observations (Rxn or No Rxn). S or support and R for Refute Cations Anions Cl- I- NO3- SO4-2 Na+(∆G°rxn) Na+ (∆Grxn) Na+ (ppt or no ppt) Na+ (Rxn or No Rxn) NO RXN NO RXN NO RXN NO RXN Na+ (S or support and R for Refute)Determine the standard enthalpy of formation ∆f Hº (in kJ/mol) for HI (g) given that the enthalpy of thereaction 2 HI (g) ---> H2 (g) + I2 (s), is delta H of the reaction = - 53.0 kJmol-1
- Table 2. Gibbs Free Energies of formation (kJ), ∆G°f, for Ions in 1M Solution and Ionic Solids cation anions Cl- -131.228 I- -51.57 NO3- -108.74 SO4-2 -744.53 Ba+2 -1296.32 W2 -663.9 -796.59 -1362.2 ∆G°f of water = -237.129 kJ/mol Calculated values of ∆G°rxn and the ∆Grxn of each box, Predicted results (ppt or no ppt). Observations (Rxn or No Rxn). S or support and R for Refute Cations Anions Cl- I- NO3- SO4-2 Ba+2(∆G°rxn) Ba+2 (∆Grxn) Ba+2 (ppt or no ppt) Ba+2 (Rxn or No Rxn) NO RXN NO RXN NO RXN RXN Ba+2 (S or support and R for Refute)The following evidence was obtained from an experiment to determine the solubility of calcium chloride at room temperature. A sample of saturated calcium chloride solution was evaporated to dryness, and the mass of solid residue was measured.EvidenceVolume of solution (mL) = 15.0Mass of empty beaker (g) = 90.54Mass of beaker and residue (g) = 101.36The solubility of calcium chloride is g/100 mLChlorodifluoromethane (CHF2Cl) was widely used in the compression/cooling circuits of refrigeration or air-conditioning systems. Since the discovery that such compounds (HCFCs and CFC's) released into the atmosphere were a major cause of depletion of stratospheric ozone, newer refrigeration systems make use of certain hydrofluorocarbons (HFCs), which are degraded in the lower atmosphere, instead. Often, mixtures of such compounds are used. Suppose a sample of refrigerant gas consisting of a simple mixture of the gases pentafluoroethane (C2HF5) and difluorormethane (CH2F2) has a density of 3.06 g/L at 14 °C and 0.974 atm. Calculate the average molecular mass for this sample
- Chlorodifluoromethane (CHF2Cl) was widely used in the compression/cooling circuits of refrigeration or air-conditioning systems. Since the discovery that such compounds (HCFCs and CFC's) released into the atmosphere were a major cause of depletion of stratospheric ozone, newer refrigeration systems make use of certain hydrofluorocarbons (HFCs), which are degraded in the lower atmosphere, instead. Often, mixtures of such compounds are used. Suppose a sample of refrigerant gas consisting of a simple mixture of the gases pentafluoroethane (C2HF5) and difluorormethane (CH2F2) has a density of 1.43 g/L at 26 °C and 0.405 atm. Calculate the average molecular mass for this sample. Calculate the volume percentage of CH2F2 in the sample.Chlorodifluoromethane (CHF2Cl) was widely used in the compression/cooling circuits of refrigeration or air-conditioning systems. Since the discovery that such compounds (HCFCs and CFC's) released into the atmosphere were a major cause of depletion of stratospheric ozone, newer refrigeration systems make use of certain hydrofluorocarbons (HFCs), which are degraded in the lower atmosphere, instead. Often, mixtures of such compounds are used. Suppose a sample of refrigerant gas consisting of a simple mixture of the gases pentafluoroethane (C2HF5) and 111-trifluoroethane (C2H3F3) has a density of 2.39 g/L at 23 °C and 0.593 atm. 1. Calculate the average molecular mass for this sample. 2. Calculate the volume percentage of C2H3F3 in the sample.Chlorodifluoromethane (CHF2Cl) was widely used in the compression/cooling circuits of refrigeration or air-conditioning systems. Since the discovery that such compounds (HCFCs and CFC's) released into the atmosphere were a major cause of depletion of stratospheric ozone, newer refrigeration systems make use of certain hydrofluorocarbons (HFCs), which are degraded in the lower atmosphere, instead. Often, mixtures of such compounds are used. Suppose a sample of refrigerant gas consisting of a simple mixture of the gases pentafluoroethane (C2HF5) and 111- trifluoroethane ((C2H3F3) has a density of1.95 g/L at 23 °C and 0.432 atm. Calculate the average molecular mass for this sample. Calculate the volume percentage of C2H3F3 in the sample.
- Chlorodifluoromethane (CHF2Cl) was widely used in the compression/cooling circuits of refrigeration or air-conditioning systems. Since the discovery that such compounds (HCFCs and CFC's) released into the atmosphere were a major cause of depletion of stratospheric ozone, newer refrigeration systems make use of certain hydrofluorocarbons (HFCs), which are degraded in the lower atmosphere, instead. Often, mixtures of such compounds are used. Suppose a sample of refrigerant gas consisting of a simple mixture of the gases pentafluoroethane (C2HF5) and difluorormethane (CH2F2) has a density of 2.99 g/L at 22 °C and 0.810 atm. Calculate the average molecular mass for this sample.8.94×101 amu Calculate the volume percentage of CH2F2 in the sample.Chlorodifluoromethane (CHF2Cl) was widely used in the compression/cooling circuits of refrigeration or air-conditioning systems. Since the discovery that such compounds (HCFCs and CFC's) released into the atmosphere were a major cause of depletion of stratospheric ozone, newer refrigeration systems make use of certain hydrofluorocarbons (HFCs), which are degraded in the lower atmosphere, instead. Often, mixtures of such compounds are used. Suppose a sample of refrigerant gas consisting of a simple mixture of the gases pentafluoroethane (C2HF5) and difluorormethane (CH2F2) has a density of 3.06 g/L at 14 °C and 0.974 atm. Calculate the volume percentage of CH2F2 in the sample.A public water supply was found to contain 0.8 partper billion (ppb) by mass of chloroform, .(a) How many molecules would be present in a350 mL glass of this water? (b) If the in part (a)could be isolated, would this quantity be detectable onan ordinary analytical balance that measures masswith a precision of g?

