The enthalpy of combustion and the standard enthalpy of formation of a fuel can be determined by measuring the temperature change in a calorimeter when a weighed amount of the fuel is burned in oxygen. (a) Write a balanced chemical equation for the combus- tion of isooctane, C3H18(€), to CO2(g) and H2O(€). Isooctane is a component of gasoline and is used as a reference standard in determining the “octane rating" of a fuel mixture. (b) Suppose 0.542 g isooctane is placed in a fixed-volume (bomb) calorimeter, which contains 750 g water, ini- tially at 20.450°C, surrounding the reaction compart- ment. The heat capacity of the calorimeter itself (excluding the water) has been measured to be 48 J K-1 in a separate calibration. After the combustion of the isooctane is complete, the water temperature is mea- sured to be 28.670°C. Taking the specific he: to be 4.184 J K-1g¬!, calculate AU for the combustion of 0.542 g isooctane. (c) Calculate AU for the combustion of 1 mol isooctane. (d) Calculate AH for the combustion of 1 mol isooctane. (e) Calculate AH°; for the isooctane.
The enthalpy of combustion and the standard enthalpy of formation of a fuel can be determined by measuring the temperature change in a calorimeter when a weighed amount of the fuel is burned in oxygen. (a) Write a balanced chemical equation for the combus- tion of isooctane, C3H18(€), to CO2(g) and H2O(€). Isooctane is a component of gasoline and is used as a reference standard in determining the “octane rating" of a fuel mixture. (b) Suppose 0.542 g isooctane is placed in a fixed-volume (bomb) calorimeter, which contains 750 g water, ini- tially at 20.450°C, surrounding the reaction compart- ment. The heat capacity of the calorimeter itself (excluding the water) has been measured to be 48 J K-1 in a separate calibration. After the combustion of the isooctane is complete, the water temperature is mea- sured to be 28.670°C. Taking the specific he: to be 4.184 J K-1g¬!, calculate AU for the combustion of 0.542 g isooctane. (c) Calculate AU for the combustion of 1 mol isooctane. (d) Calculate AH for the combustion of 1 mol isooctane. (e) Calculate AH°; for the isooctane.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 75AP
Related questions
Question
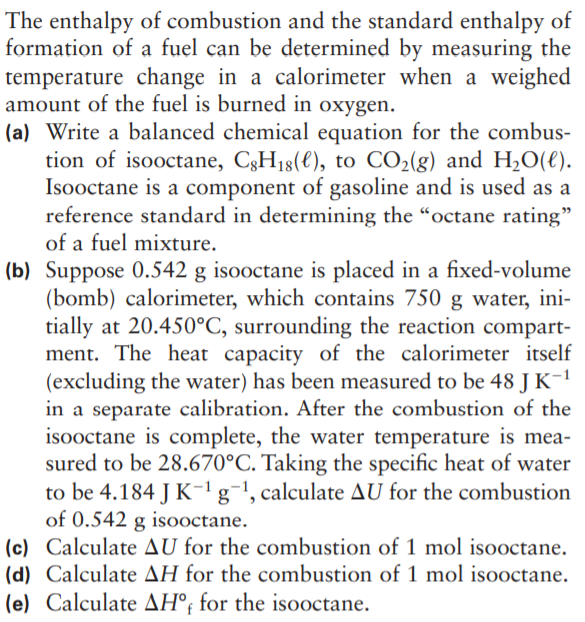
Transcribed Image Text:The enthalpy of combustion and the standard enthalpy of
formation of a fuel can be determined by measuring the
temperature change in a calorimeter when a weighed
amount of the fuel is burned in oxygen.
(a) Write a balanced chemical equation for the combus-
tion of isooctane, C3H18(€), to CO2(g) and H2O(€).
Isooctane is a component of gasoline and is used as a
reference standard in determining the “octane rating"
of a fuel mixture.
(b) Suppose 0.542 g isooctane is placed in a fixed-volume
(bomb) calorimeter, which contains 750 g water, ini-
tially at 20.450°C, surrounding the reaction compart-
ment. The heat capacity of the calorimeter itself
(excluding the water) has been measured to be 48 J K-1
in a separate calibration. After the combustion of the
isooctane is complete, the water temperature is mea-
sured to be 28.670°C. Taking the specific he:
to be 4.184 J K-1g¬!, calculate AU for the combustion
of 0.542 g isooctane.
(c) Calculate AU for the combustion of 1 mol isooctane.
(d) Calculate AH for the combustion of 1 mol isooctane.
(e) Calculate AH°; for the isooctane.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning