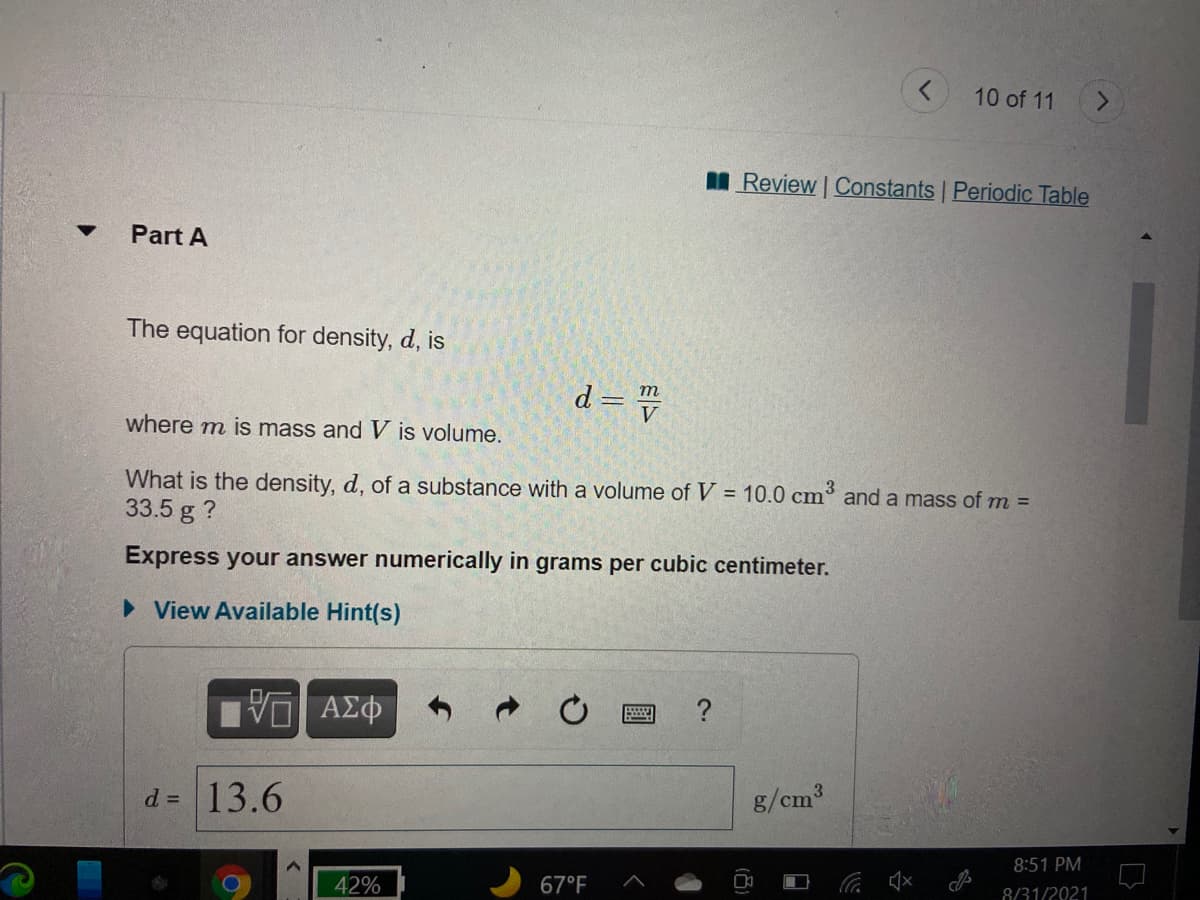
The equation for density, d, is d = v m where m is mass and V is volume. 3 What is the density, d, of a substance with a volume of V = 10.0 cm and a mass of m = 33.5 g ? %3D Express your answer numerically in grams per cubic centimeter. • View Available Hint(s)
The equation for density, d, is d = v m where m is mass and V is volume. 3 What is the density, d, of a substance with a volume of V = 10.0 cm and a mass of m = 33.5 g ? %3D Express your answer numerically in grams per cubic centimeter. • View Available Hint(s)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter18: Representative Metals, Metalloids, And Nonmetals
Section: Chapter Questions
Problem 4E: Predict the best choice in each of the following. You may wish to review the Chapter on electronic...
Related questions
Question
Transcribed Image Text:10 of 11
<.
I Review | Constants Periodic Table
Part A
The equation for density, d, is
d = =
m
where m is mass and V is volume.
What is the density, d, of a substance with a volume of V = 10.0 cm
33.5 g ?
and a mass of m =
Express your answer numerically in grams per cubic centimeter.
• View Available Hint(s)
ΑΦ
?
d = 13.6
g/cm3
8:51 PM
42%
67°F
8/31/2021
Transcribed Image Text:How to Calcula
nVellumHMAC=20ff3692a9b8f8628f99c8c00d38dabd#10301
ps
Translate
EReading list
I Review | Constants Periodic Table
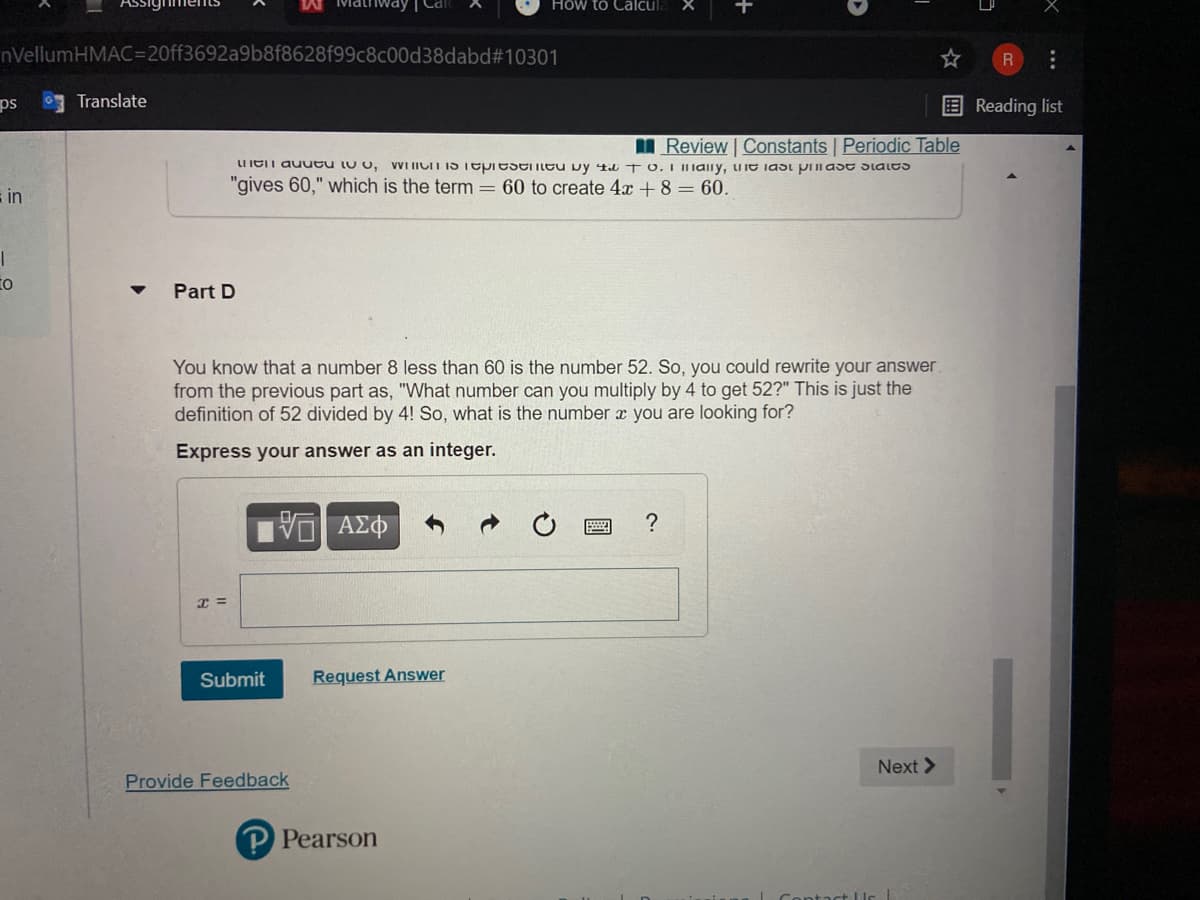
VWI IICIT IS Tepiesentu vy 4a T O.I IaIly, uie iast prlast Slales
UIEIT auueu lu o,
"gives 60," which is the term = 60 to create 4x + 8 = 60.
in
to
Part D
You know that a number 8 less than 60 is the number 52. So, you could rewrite your answer.
from the previous part as, "What number can you multiply by 4 to get 52?" This is just the
definition of 52 divided by 4! So, what is the number x you are looking for?
Express your answer as an integer.
V AZO
?
Submit
Request Answer
Next >
Provide Feedback
P Pearson
...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning