The equilibrium potential for a given ion (Eion) is a theoretical value. For a given concentration gradient of an ion, the equilibrium potential is the charge inside the cell required to hold an ion at that concentration. That is, it is the charge required to perfectly oppose the drive of the ion to move down its concentration gradient. So, if the concentration of Nat is higher outside the cell than inside, its equilibrium potential (ENa) must be I and if we add more sodium to the extracellular fluid, then ENa will II.
The equilibrium potential for a given ion (Eion) is a theoretical value. For a given concentration gradient of an ion, the equilibrium potential is the charge inside the cell required to hold an ion at that concentration. That is, it is the charge required to perfectly oppose the drive of the ion to move down its concentration gradient. So, if the concentration of Nat is higher outside the cell than inside, its equilibrium potential (ENa) must be I and if we add more sodium to the extracellular fluid, then ENa will II.
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter32: The Reception And Transmission Of Extracellular Information
Section: Chapter Questions
Problem 14P
Related questions
Question
The equilibrium potential for a given ion (Eion) is a theoretical value. For a given concentration gradient of an ion, the equilibrium potential is the charge inside the cell required to hold an ion at that concentration. That is, it is the charge required to perfectly oppose the drive of the ion to move down its concentration gradient. So, if the concentration of Nat is higher outside the cell than inside, its equilibrium potential (ENa) must be I and if we add more sodium to the extracellular fluid, then ENa will II.
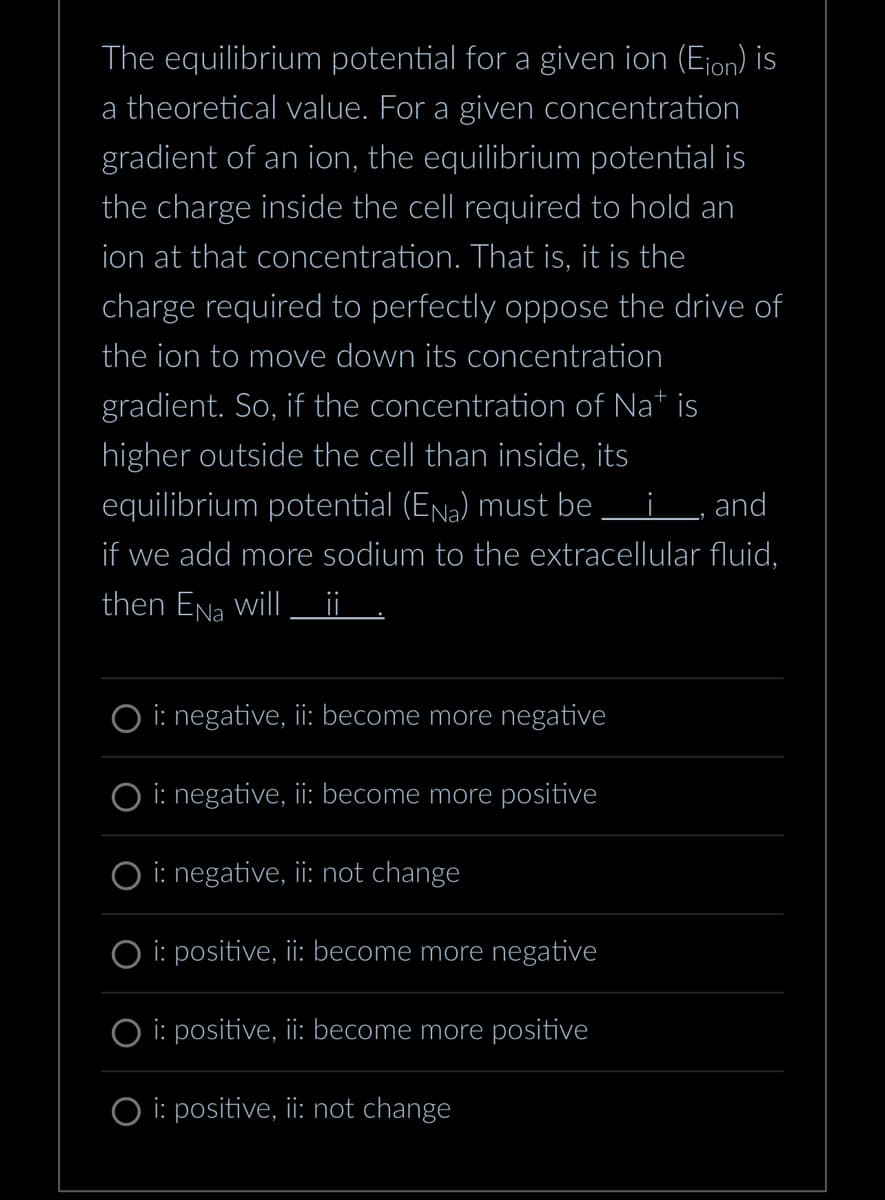
Transcribed Image Text:The equilibrium potential for a given ion (Eion) is
a theoretical value. For a given concentration
gradient of an ion, the equilibrium potential is
the charge inside the cell required to hold an
ion at that concentration. That is, it is the
charge required to perfectly oppose the drive of
the ion to move down its concentration
gradient. So, if the concentration of Nat is
higher outside the cell than inside, its
equilibrium potential (Ena) must be i, and
if we add more sodium to the extracellular fluid,
then ENa will ¡¡
O i: negative, ii: become more negative
i: negative, ii: become more positive
O i: negative, ii: not change
Oi: positive, ii: become more negative
i: positive, ii: become more positive
Oi: positive, ii: not change
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning