The following data was obtained during the alar Mass of Magnasium simula Initial pressure reading (atm) 1.000 Initial temperature reading ("C) 21.5 0.085 maximum pressure reading (atm) maximum temperature reading ("C) 28.4 final pressure reading (atm) 1.270 final temperature reading (C) 21.5 visual observations upon adding Bubbling gas magnesium volume of H, (g) in syringe (mL) 78.41 pressure of Hz Ig) in syringe 1.000 atm What is the molar mass of magnesium? PV nRT 1 atm 760 mm Hg n-g/MW OC 273.15 K Select one: O24 g/mol
The following data was obtained during the alar Mass of Magnasium simula Initial pressure reading (atm) 1.000 Initial temperature reading ("C) 21.5 0.085 maximum pressure reading (atm) maximum temperature reading ("C) 28.4 final pressure reading (atm) 1.270 final temperature reading (C) 21.5 visual observations upon adding Bubbling gas magnesium volume of H, (g) in syringe (mL) 78.41 pressure of Hz Ig) in syringe 1.000 atm What is the molar mass of magnesium? PV nRT 1 atm 760 mm Hg n-g/MW OC 273.15 K Select one: O24 g/mol
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.39E: A refrigerator contains approximately 17cubic feet, or about 480 liters, of air. Assuming it acts as...
Related questions
Question
100%
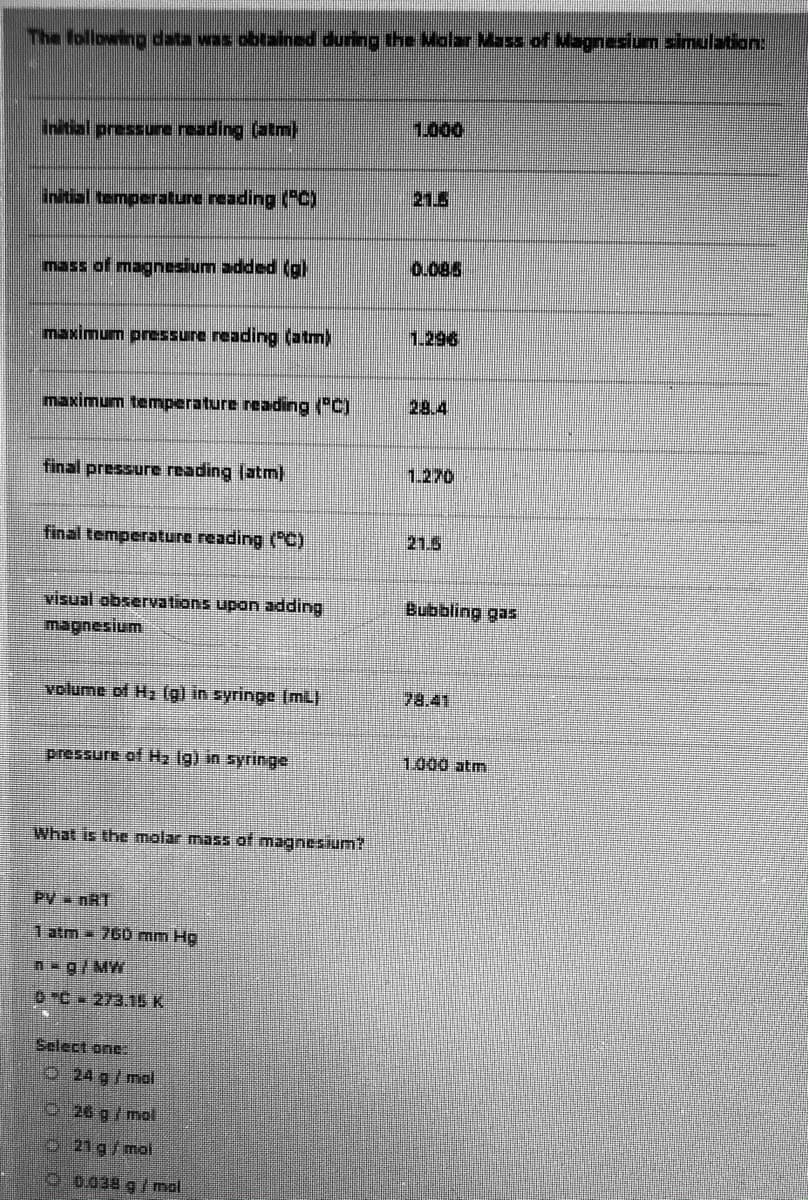
Transcribed Image Text:The following data was obtained during the lalar Mass of agnasium simulation:
1,000
(ur) Bupe anssad
Initial temperature reading ("C)
21.5
mass of magnesium added (g)
0.085
maximum pressure reading (atm)
1.296
maximum temperature reading ("C)
28.4
final pressure reading (atm)
1.270
final temperature reading (C)
21.5
visual observations upon adding
seb Bung
volume of H, (g) in syringe (mL)
78.41
pressure of Hz Ig) in syringe
1.000 atm
What is the molar mass of magnesium?
PV nRT
1 atm 760 mm Hg
n-g/MW
DC 273.15K
Select one
24 g/ mol
O26 g/mol
21g/mol
00.038 g/ mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning