The following data was obtained for an experiment on the gas chromatographic determination of toluene in an unknown sample using the internal standard method. Concentration (mass %) Toluene Peak Area Ethylbenzene Peak Area 0.50 0.539 1.970 1.00 1.150 2.051 2.00 2.174 2.018 3.00 3.308 2.009 unknown 1.382 2.015 a) Plot the calibration curve for this analysis and calculate the slope, y-intercept, and Pearson correlation coefficient. b) Find the % of toluene in the unknown.
The following data was obtained for an experiment on the gas chromatographic determination of toluene in an unknown sample using the internal standard method. Concentration (mass %) Toluene Peak Area Ethylbenzene Peak Area 0.50 0.539 1.970 1.00 1.150 2.051 2.00 2.174 2.018 3.00 3.308 2.009 unknown 1.382 2.015 a) Plot the calibration curve for this analysis and calculate the slope, y-intercept, and Pearson correlation coefficient. b) Find the % of toluene in the unknown.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter26: An Introduction To Chromatographic Separations
Section: Chapter Questions
Problem 26.14QAP
Related questions
Question
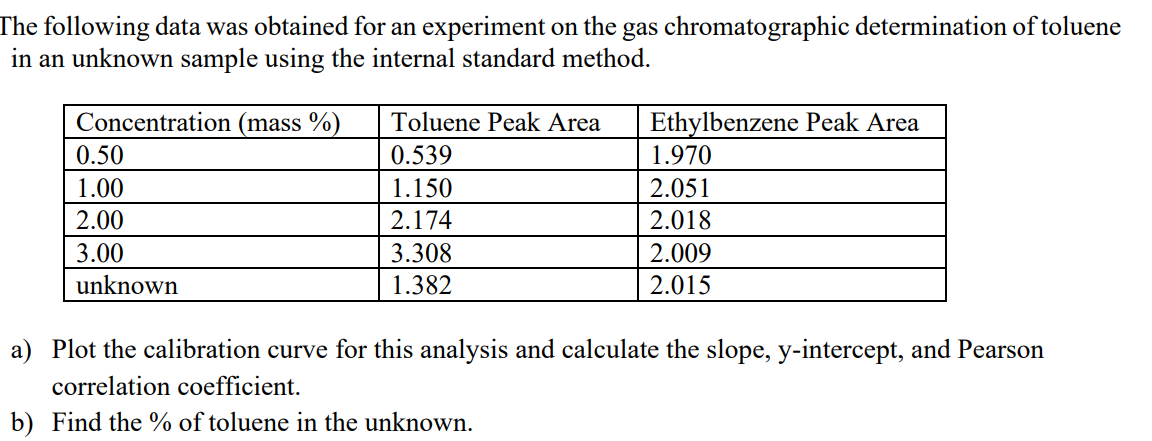
Transcribed Image Text:The following data was obtained for an experiment on the gas chromatographic determination of toluene
in an unknown sample using the internal standard method.
Concentration (mass %)
Toluene Peak Area
Ethylbenzene Peak Area
0.50
0.539
1.970
1.00
1.150
2.051
2.00
2.174
2.018
3.00
3.308
2.009
unknown
1.382
2.015
a) Plot the calibration curve for this analysis and calculate the slope, y-intercept, and Pearson
correlation coefficient.
b) Find the % of toluene in the unknown.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT