The following data were obtained at various wavelengths for a 3.6 ´ 10–4 M solution of permanganate in a cell with a path length of 1.00 cm. (Table is in attached) (a) Plot an absorption spectrum for permanganate using these data. (b) From the absorption spectrum obtained, determine the wavelength that permanganate has the strongest absorption. (c) Estimate the molar absorptivity (with proper unit) of permanganate at the absorption maximum. (d) A sample solution containing permanganate gives a %T of 40.0 at the absorption maximum. What is the concentration of permanganate in the sample?
The following data were obtained at various wavelengths for a 3.6 ´ 10–4 M solution of permanganate in a cell with a path length of 1.00 cm. (Table is in attached) (a) Plot an absorption spectrum for permanganate using these data. (b) From the absorption spectrum obtained, determine the wavelength that permanganate has the strongest absorption. (c) Estimate the molar absorptivity (with proper unit) of permanganate at the absorption maximum. (d) A sample solution containing permanganate gives a %T of 40.0 at the absorption maximum. What is the concentration of permanganate in the sample?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter19: Nuclear Magnetic Resonance Spectroscopy
Section: Chapter Questions
Problem 19.14QAP: What is a rotating frame of reference?
Related questions
Question
3. The following data were obtained at various wavelengths for a 3.6 ´ 10–4 M solution of permanganate in a cell with a path length of 1.00 cm.
(Table is in attached)
|
(a) |
Plot an absorption spectrum for permanganate using these data. |
|
(b) |
From the absorption spectrum obtained, determine the wavelength that permanganate has the strongest absorption. |
|
(c) |
Estimate the molar absorptivity (with proper unit) of permanganate at the absorption maximum. |
|
(d) |
A sample solution containing permanganate gives a %T of 40.0 at the absorption maximum. What is the concentration of permanganate in the sample? |
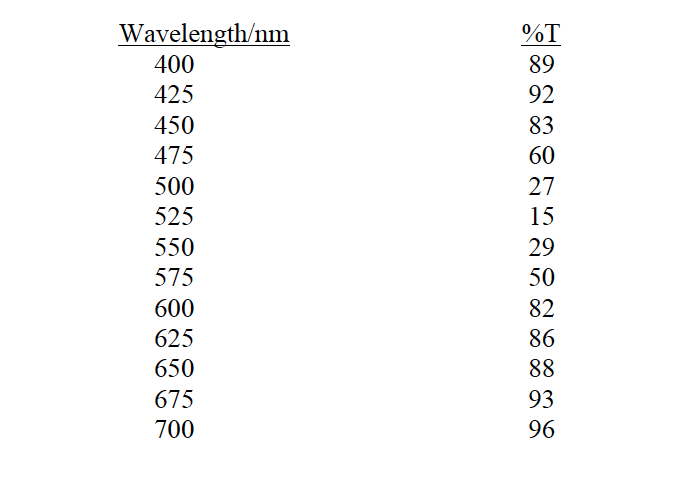
Transcribed Image Text:Wavelength/nm
%T
400
89
425
92
450
83
475
60
500
27
525
15
550
29
575
50
600
82
625
86
650
88
675
93
700
96
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning