The following sets of quantum numbers, listed in the order n, l, mų, and ms, were written for the last electrons added to an atom. Identify which sets are valid and classify the others by the rule or principle that is violated. Drag the appropriate items to their respective bins. > View Availahle Hint(s)
The following sets of quantum numbers, listed in the order n, l, mų, and ms, were written for the last electrons added to an atom. Identify which sets are valid and classify the others by the rule or principle that is violated. Drag the appropriate items to their respective bins. > View Availahle Hint(s)
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section: Chapter Questions
Problem 19PS: The energy emitted when an electron moves from a higher energy state to a lower energy state in any...
Related questions
Question
Please answer question 1 part A and B
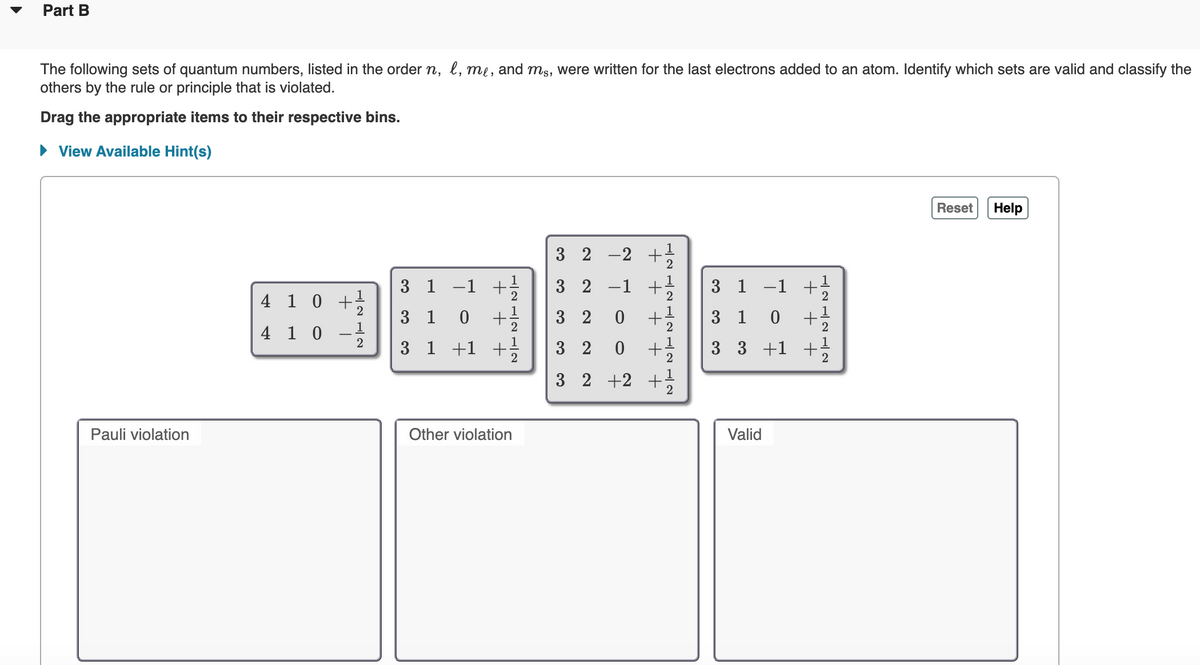
Transcribed Image Text:Part B
The following sets of quantum numbers, listed in the order n, l, me, and ms, were written for the last electrons added to an atom. Identify which sets are valid and classify the
others by the rule or principle that is violated.
Drag the appropriate items to their respective bins.
• View Available Hint(s)
Reset
Help
3 2 -2 +
3 1
-1 +
3 2 -1
3 1 -1 +
4 1 0 +:
0 +
2
3 1
1
3 2
3 1 0
2
2
4 1 0
-
2
3 1 +1 +
3 2
3 3 +1 +
2
3 2 +2 +
1
Pauli violation
Other violation
Valid
2.
|N -| N 1|
+ +
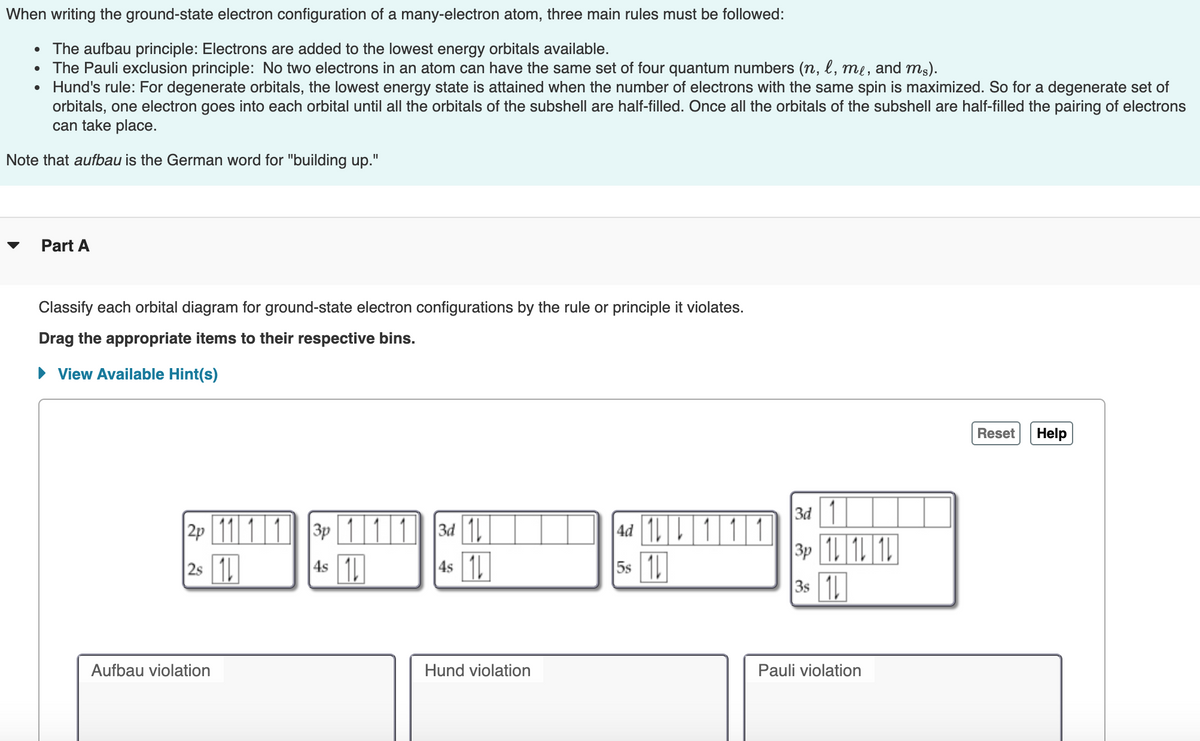
Transcribed Image Text:When writing the ground-state electron configuration of a many-electron atom, three main rules must be followed:
• The aufbau principle: Electrons are added to the lowest energy orbitals available.
• The Pauli exclusion principle: No two electrons in an atom can have the same set of four quantum numbers (n, l, me, and ms).
• Hund's rule: For degenerate orbitals, the lowest energy state is attained when the number of electrons with the same spin is maximized. So for a degenerate set of
orbitals, one electron goes into each orbital until all the orbitals of the subshell are half-filled. Once all the orbitals of the subshell are half-filled the pairing of electrons
can take place.
Note that aufbau is the German word for "building up."
Part A
Classify each orbital diagram for ground-state electron configurations by the rule or principle it violates.
Drag the appropriate items to their respective bins.
• View Available Hint(s)
Reset
Help
|2P 1111 3 111 34 1||
4s 1
3d 1
3p 1 1 1
|4d 1|
2s 1
|4s 1
5s 1
|3s 1
Aufbau violation
Hund violation
Pauli violation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning